In 2025, Samsung Biologics continued its steady growth across key business areas. As the year draws to a close, the following highlights the key developments this past year—from the launch of new platforms and the opening of Bio Campus II to the introduction of ExellenS™, an optimized manufacturing framework which reinforces Samsung Biologics’ advantage for clients and ultimately for patients. The company also announced a defining step in its geographical expansion strategy with the planned acquisition of a U.S. facility which is expected to close at the end of Q1, 2026.
Continuous Pursuit of Excellence
Samsung Biologics strengthened its development and manufacturing capabilities to accommodate a wide range of modalities and client needs.
.webp)
The launch of Samsung Organoids advanced research and discovery through three-dimensional cancer models that closely reflect human biology. By supporting more informed decision-making in early development stages, the platform contributes to clearer trajectories across diverse programs.
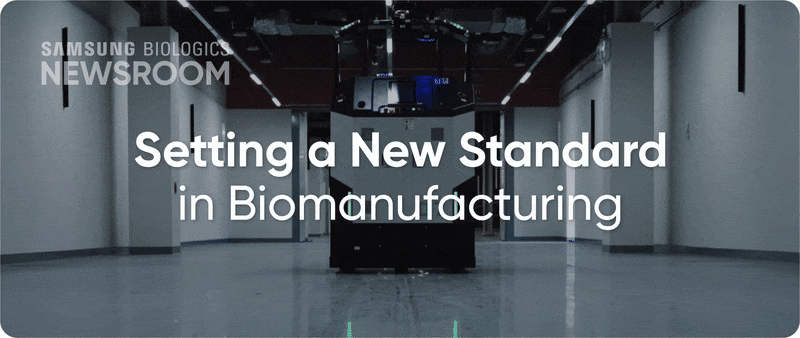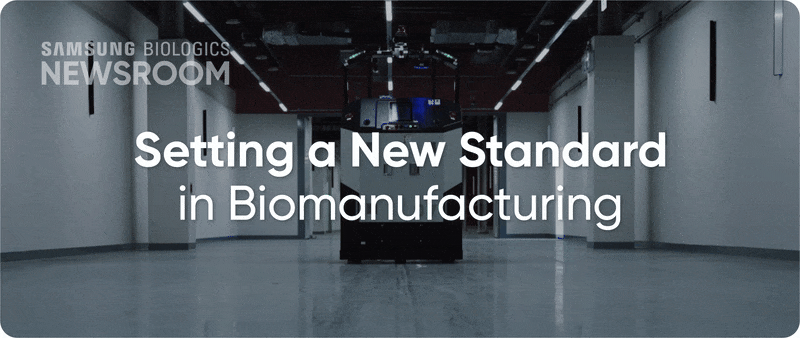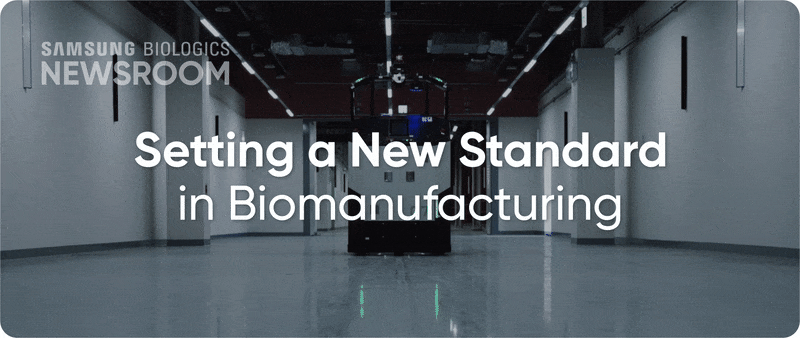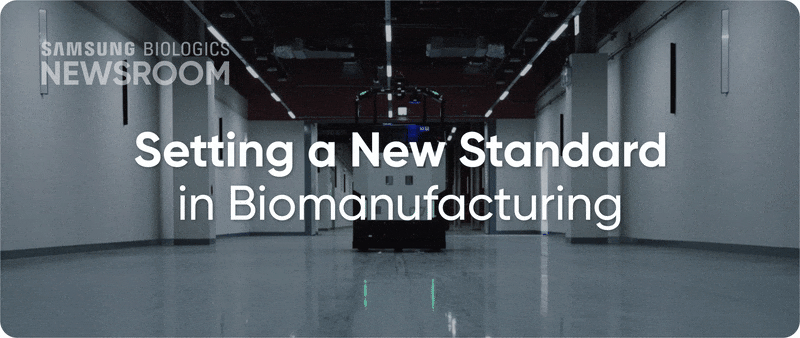
The completion of Plant 5 marked the beginning of Bio Campus II, showcasing in record time, capacity growth with unmatched digitalization. Plant 5 not only meets established operational standards reflecting diverse client needs, it also integrates innovative infrastructure to support next-generation processes with agility and efficiency.
▶ Plant 5: Re-shaping biopharma operations

Samsung Biologics announced its first U.S.-based manufacturing site through the planned acquisition of a facility in Rockville, Maryland, from GSK. This strategic move will add 60,000 liters of capacity upon deal closure at the end of Q1 2026, supporting the company’s continued efforts to strengthen manufacturing capabilities and operational flexibility for clients.

Another milestone was the introduction of ExellenS™, an optimized manufacturing framework designed for operational consistency. Built on the company’s four pillars of excellence over years of proven success, the framework provides a structural basis for stable execution and unmatched quality in manufacturing operations.
▶ ExellenS™: Samsung Biologics’ optimized manufacturing framework
Expertise in Reliable Execution
Scientists and engineers at Samsung Biologics drew on their experience across diverse modalities and production stages to share their perspectives on development and manufacturing strategies. These insights addressed the practical challenges associated with simplifying processes and streamlining programs for complex molecules. By applying technical expertise to real-world program needs, Samsung Biologics maintained its focus on supporting reliable execution and long-term project success.
▶ Enabling mRNA advances through collaborative technologies
▶ Empowering clients for multispecific antibody therapeutics
▶ Agile and reliable technology transfer for complex modalities
▶ Ask Our Experts: Navigating the complexities of drug development with integrated platforms

Collaboration with Clients
Client relationships remained central to our operations this year. Testimonials shared by our clients reflected how collaborative approaches accelerate timelines, sustain partnerships, and facilitate problem-solving across complex projects. This ongoing collaboration reinforces consistent project delivery while maintaining operational rigor.
▶ Powering progress through partnership: CDMO collaboration for fast-tracked success
▶ Driving a decade of partnerships

Our People and Culture Behind the Expansion
Underpinning the strong progress made this year were the people and culture that drive daily operations. Stories marking occasions such as International Women’s Day and World Quality Week offered insights into the perspectives, experiences, and teamwork across the organization.

These moments highlighted how meaningful engagement, professional growth, and a shared quality mindset contribute to consistent performance and continuous refinement as capabilities evolve.
▶ People Excellence: Driver of Samsung Biologics’ growth
▶ Quality mindset cultivated at Samsung Biologics
▶ Quality mindset ingrained in operations for client excellence
▶ Empowering women in biopharma: Advancing quality through diversity
▶ World Quality Week 2025 | Fostering a culture of quality
▶ Enhancing collaborative communication for stronger teams
▶ Bringing Families Closer: A Shared Journey of Care, Commitment, and Mission

As Samsung Biologics continues its growth and momentum, the focus remains on strengthening its capabilities across development and manufacturing to best support client programs across the life sciences industry. The company will continue to advance its operations and services to benefit clients and ultimately patients worldwide.
In 2025, Samsung Biologics continued its steady growth across key business areas. As the year draws to a close, the following highlights the key developments this past year—from the launch of new platforms and the opening of Bio Campus II to the introduction of ExellenS™, an optimized manufacturing framework which reinforces Samsung Biologics’ advantage for clients and ultimately for patients. The company also announced a defining step in its geographical expansion strategy with the planned acquisition of a U.S. facility which is expected to close at the end of Q1, 2026.
Continuous Pursuit of Excellence
Samsung Biologics strengthened its development and manufacturing capabilities to accommodate a wide range of modalities and client needs.

The launch of Samsung Organoids advanced research and discovery through three-dimensional cancer models that closely reflect human biology. By supporting more informed decision-making in early development stages, the platform contributes to clearer trajectories across diverse programs.
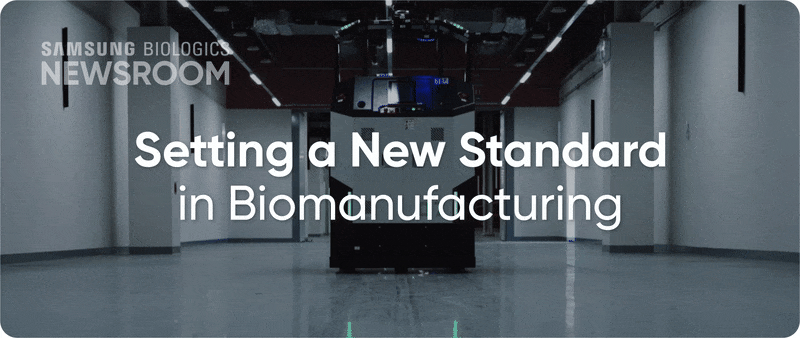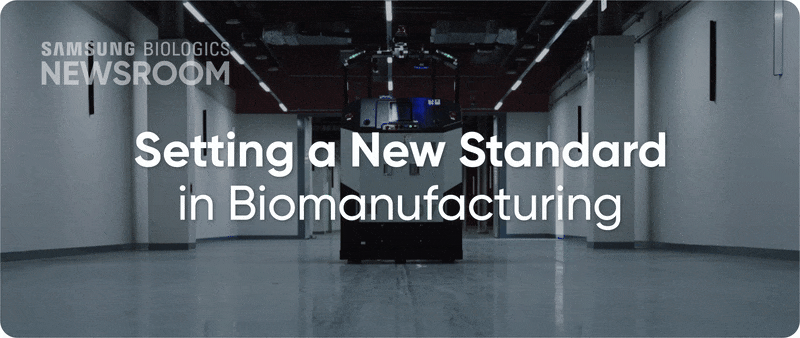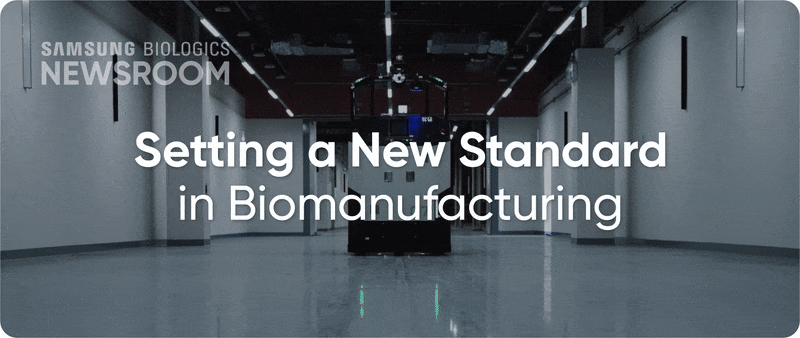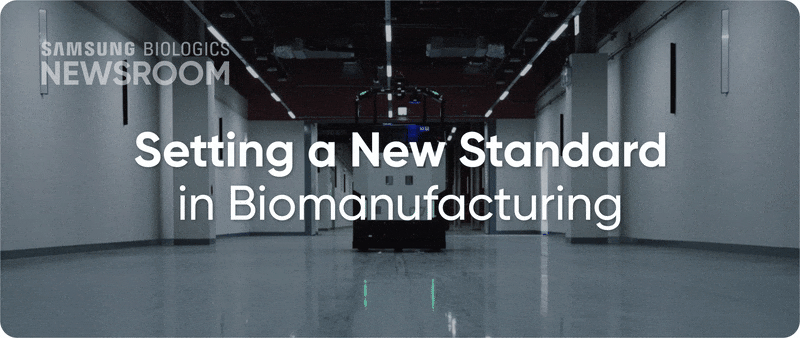
The completion of Plant 5 marked the beginning of Bio Campus II, showcasing in record time, capacity growth with unmatched digitalization. Plant 5 not only meets established operational standards reflecting diverse client needs, it also integrates innovative infrastructure to support next-generation processes with agility and efficiency.
▶ Plant 5: Re-shaping biopharma operations

Samsung Biologics announced its first U.S.-based manufacturing site through the planned acquisition of a facility in Rockville, Maryland, from GSK. This strategic move will add 60,000 liters of capacity upon deal closure at the end of Q1 2026, supporting the company’s continued efforts to strengthen manufacturing capabilities and operational flexibility for clients.

Another milestone was the introduction of ExellenS™, an optimized manufacturing framework designed for operational consistency. Built on the company’s four pillars of excellence over years of proven success, the framework provides a structural basis for stable execution and unmatched quality in manufacturing operations.
▶ ExellenS™: Samsung Biologics’ optimized manufacturing framework
Expertise in Reliable Execution
Scientists and engineers at Samsung Biologics drew on their experience across diverse modalities and production stages to share their perspectives on development and manufacturing strategies. These insights addressed the practical challenges associated with simplifying processes and streamlining programs for complex molecules. By applying technical expertise to real-world program needs, Samsung Biologics maintained its focus on supporting reliable execution and long-term project success.
▶ Enabling mRNA advances through collaborative technologies
▶ Empowering clients for multispecific antibody therapeutics
▶ Agile and reliable technology transfer for complex modalities
▶ Ask Our Experts: Navigating the complexities of drug development with integrated platforms

Collaboration with Clients
Client relationships remained central to our operations this year. Testimonials shared by our clients reflected how collaborative approaches accelerate timelines, sustain partnerships, and facilitate problem-solving across complex projects. This ongoing collaboration reinforces consistent project delivery while maintaining operational rigor.
▶ Powering progress through partnership: CDMO collaboration for fast-tracked success
▶ Driving a decade of partnerships

Our People and Culture Behind the Expansion
Underpinning the strong progress made this year were the people and culture that drive daily operations. Stories marking occasions such as International Women’s Day and World Quality Week offered insights into the perspectives, experiences, and teamwork across the organization.

These moments highlighted how meaningful engagement, professional growth, and a shared quality mindset contribute to consistent performance and continuous refinement as capabilities evolve.
▶ People Excellence: Driver of Samsung Biologics’ growth
▶ Quality mindset cultivated at Samsung Biologics
▶ Quality mindset ingrained in operations for client excellence
▶ Empowering women in biopharma: Advancing quality through diversity
▶ World Quality Week 2025 | Fostering a culture of quality
▶ Enhancing collaborative communication for stronger teams
▶ Bringing Families Closer: A Shared Journey of Care, Commitment, and Mission

As Samsung Biologics continues its growth and momentum, the focus remains on strengthening its capabilities across development and manufacturing to best support client programs across the life sciences industry. The company will continue to advance its operations and services to benefit clients and ultimately patients worldwide.
Share article
Related Content