End-to-End Drug Substance Manufacturing
Modalities
CGMP Cell Culture Capabilities
-
Commercial
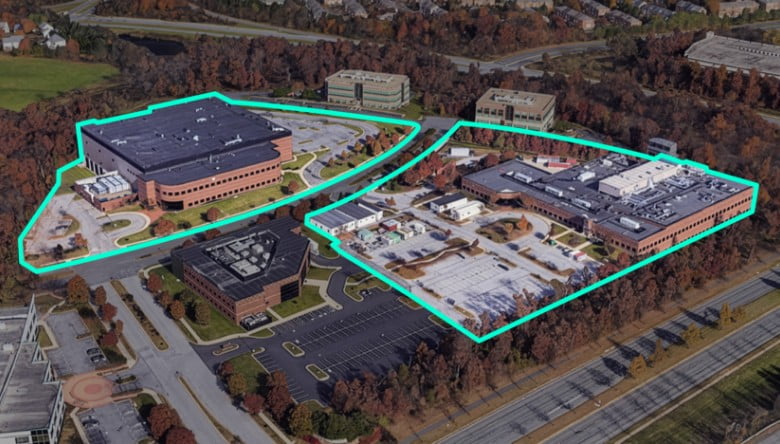
- As one of the top biologics CDMO by production capacity, Samsung Biologics specializes in large-scale biologics manufacturing with a focus on commercial mammalian cell culture services. Our advanced production suites are equipped with chrmoatography columns, UF/DF skids, virus filtration skids, product pool vessels, and Grade A bulk filling capability to address complex process demands. With flexible bioreactor sizes, Samsung Biologics collaborates with clients throughout the entire product lifecycle, ensuring efficient and reliable drug substance manufacturing and drug product manufacturing for seamless drug supply management. Discover our Biopharma Manufacturing capabilities.
-
Mid to Late-stage Clinical
- Samsung Biologics offers comprehensive CGMP manufacturing services tailored to mid- to late-stage clinical development. Our versatile bioreactor capacities, state-of-the-art technologies, and deep technical expertise make us a reliable partner for clinical manufacturing and process characterization. We support all pre-BLA activities and provide integrated post-launch lifecycle management, efficiently addressing commercial and CMC requirements. Learn about our Process Development and Analytical Development services designed to accelerate your biologics development timeline.
Facility
-
- kL
- Total Capacity
-
- %
- Batch success rate
Related Content