Due to an immunosuppressive tumor microenvironment and the presence of more than one biomarker in cancer cells, multispecific antibodies (msAbs) can serve as a powerful therapeutic for their ability to target multiple antigens.
The msAb manufacturing process, however, can be complex – an additional ultrafiltration and diafiltration step means purification can take 1.5 to 2 times longer than for monoclonal antibodies (mAbs). To navigate the complexity in conducting experiments and evaluating the data, analytical expertise is also essential.
Tech transfer and development platform expertise
Samsung Biologics offers flexible services catered to meet unique client needs and molecules including msAbs, backed by its expertise in tech transfer for mAb development and manufacturing as a leading CDMO with a track record of success. "Samsung Biologics has extensive know-how in mAb development and manufacturing," said Professor Sukmook Lee of Kookmin University and member of the Samsung Biologics Biotechnology Advisory Board.
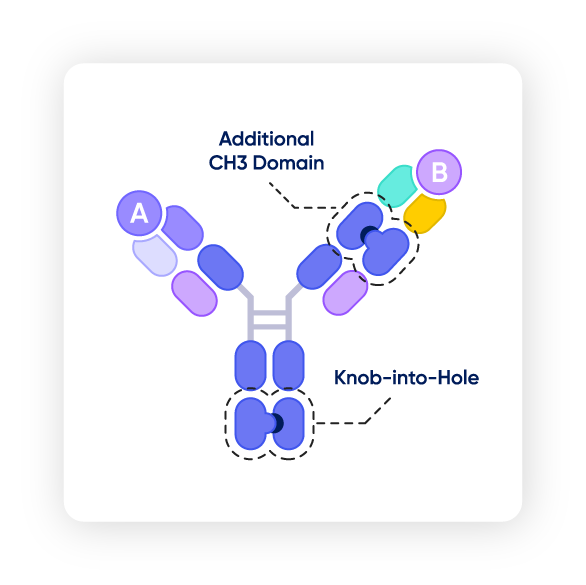 ▲ The S-DUAL™ platform
▲ The S-DUAL™ platform
For clients looking for optimal purity, titer, and solubility for their molecules, the right CDMO partner can offer advanced platforms to accommodate these needs. The S-DUAL™ platform achieves up to 99% purity alongside high solubility, while preventing chain mispairing. Samsung Biologics’ development arm working closely with its manufacturing team creates a seamless, end-to-end experience for clients.
Among seasoned in-house researchers at Samsung Biologics, proactive communication streamlines resources while also helping to attain optimal purity and titer. MsAb development and manufacturing are complex projects for which the CDMO’s capable scientists and operators are well-suited.
Powered by technical understanding
Together with the expertise in addressing the relative unpredictability of cell culture and purification steps for msAbs compared to mAbs, in-depth knowledge about resins during analytical experiments exemplify the CDMO’s scientific culture. Samsung Biologics’ commitment to comprehensive resin screening and a variety of analytical tools ensure the high quality of resulting complex molecules. In silico analysis of protein structure empowers scientists to elucidate the cause of charge or hydrophobicity issues during development. “We are internalizing the use of AI-powered technology to predict the structure and characteristics of antibodies,” said Yeumin Kim, Senior Scientist of Antibody Technology Application.
Through continued dedication to basic research, the biopharma industry including CDMOs can further understand the nature of biomarkers and adapt new technologies. “Samsung Biologics has the expertise to evaluate msAbs’ complex proof of concept relative to that of mAbs,” said Sangho Lee, Senior Scientist of Antibody Technology Discovery.
Production schedule flexibility and synergy
Samsung Biologics’ production schedule flexibility has also enabled the successful adaptation of theoretical knowledge to yield concrete results when producing msAbs from parental antibodies. “Clients have expressed their thanks,” said Joomyung Lee, Lead Engineer and Director of Purification.
As one team, Samsung Biologics closely watches market trends to understand emerging targets and platform mechanisms, while empirically probing for mutations that improve solubility and developability. Know-how in adapting manufacturing facilities for msAb therapeutics and a continued prioritization of quality drive the global CDMO’s tech transfer and operations experts toward excellence in meeting client needs. The synergy between flexible facilities and focus on client programs positions the company to be the CDMO partner of choice.
Related content
Samsung BIO Insight Applying advanced manufacturing technology to complex biomedicines
Whitepapers Strategic biomanufacturing partner for multispecific antibodies
Due to an immunosuppressive tumor microenvironment and the presence of more than one biomarker in cancer cells, multispecific antibodies (msAbs) can serve as a powerful therapeutic for their ability to target multiple antigens.

The msAb manufacturing process, however, can be complex – an additional ultrafiltration and diafiltration step means purification can take 1.5 to 2 times longer than for monoclonal antibodies (mAbs). To navigate the complexity in conducting experiments and evaluating the data, analytical expertise is also essential.
Tech transfer and development platform expertise
Samsung Biologics offers flexible services catered to meet unique client needs and molecules including msAbs, backed by its expertise in tech transfer for mAb development and manufacturing as a leading CDMO with a track record of success. "Samsung Biologics has extensive know-how in mAb development and manufacturing," said Professor Sukmook Lee of Kookmin University and member of the Samsung Biologics Biotechnology Advisory Board.
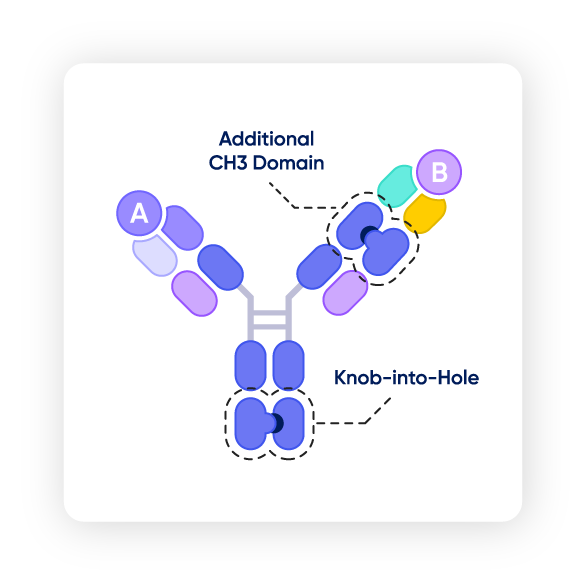
▲ The S-DUAL™ platform
For clients looking for optimal purity, titer, and solubility for their molecules, the right CDMO partner can offer advanced platforms to accommodate these needs. The S-DUAL™ platform achieves up to 99% purity alongside high solubility, while preventing chain mispairing. Samsung Biologics’ development arm working closely with its manufacturing team creates a seamless, end-to-end experience for clients.
Among seasoned in-house researchers at Samsung Biologics, proactive communication streamlines resources while also helping to attain optimal purity and titer. MsAb development and manufacturing are complex projects for which the CDMO’s capable scientists and operators are well-suited.
Powered by technical understanding
Together with the expertise in addressing the relative unpredictability of cell culture and purification steps for msAbs compared to mAbs, in-depth knowledge about resins during analytical experiments exemplify the CDMO’s scientific culture. Samsung Biologics’ commitment to comprehensive resin screening and a variety of analytical tools ensure the high quality of resulting complex molecules. In silico analysis of protein structure empowers scientists to elucidate the cause of charge or hydrophobicity issues during development. “We are internalizing the use of AI-powered technology to predict the structure and characteristics of antibodies,” said Yeumin Kim, Senior Scientist of Antibody Technology Application.
Through continued dedication to basic research, the biopharma industry including CDMOs can further understand the nature of biomarkers and adapt new technologies. “Samsung Biologics has the expertise to evaluate msAbs’ complex proof of concept relative to that of mAbs,” said Sangho Lee, Senior Scientist of Antibody Technology Discovery.
Production schedule flexibility and synergy
Samsung Biologics’ production schedule flexibility has also enabled the successful adaptation of theoretical knowledge to yield concrete results when producing msAbs from parental antibodies. “Clients have expressed their thanks,” said Joomyung Lee, Lead Engineer and Director of Purification.
As one team, Samsung Biologics closely watches market trends to understand emerging targets and platform mechanisms, while empirically probing for mutations that improve solubility and developability. Know-how in adapting manufacturing facilities for msAb therapeutics and a continued prioritization of quality drive the global CDMO’s tech transfer and operations experts toward excellence in meeting client needs. The synergy between flexible facilities and focus on client programs positions the company to be the CDMO partner of choice.
Related content
Samsung BIO Insight Applying advanced manufacturing technology to complex biomedicines
Whitepapers Strategic biomanufacturing partner for multispecific antibodies
Share article
Related Content