Standardized manufacturing design for consistent, reliable execution
Early-stage decisions are playing an increasingly important role in the execution success of biopharmaceutical programs. Facility alignment, process fit, and equipment planning considerations can significantly influence timelines, scalability, and long-term supply continuity. As a result, structured manufacturing frameworks that support consistent outcomes across the manufacturing lifecycle are essential for reliable execution.
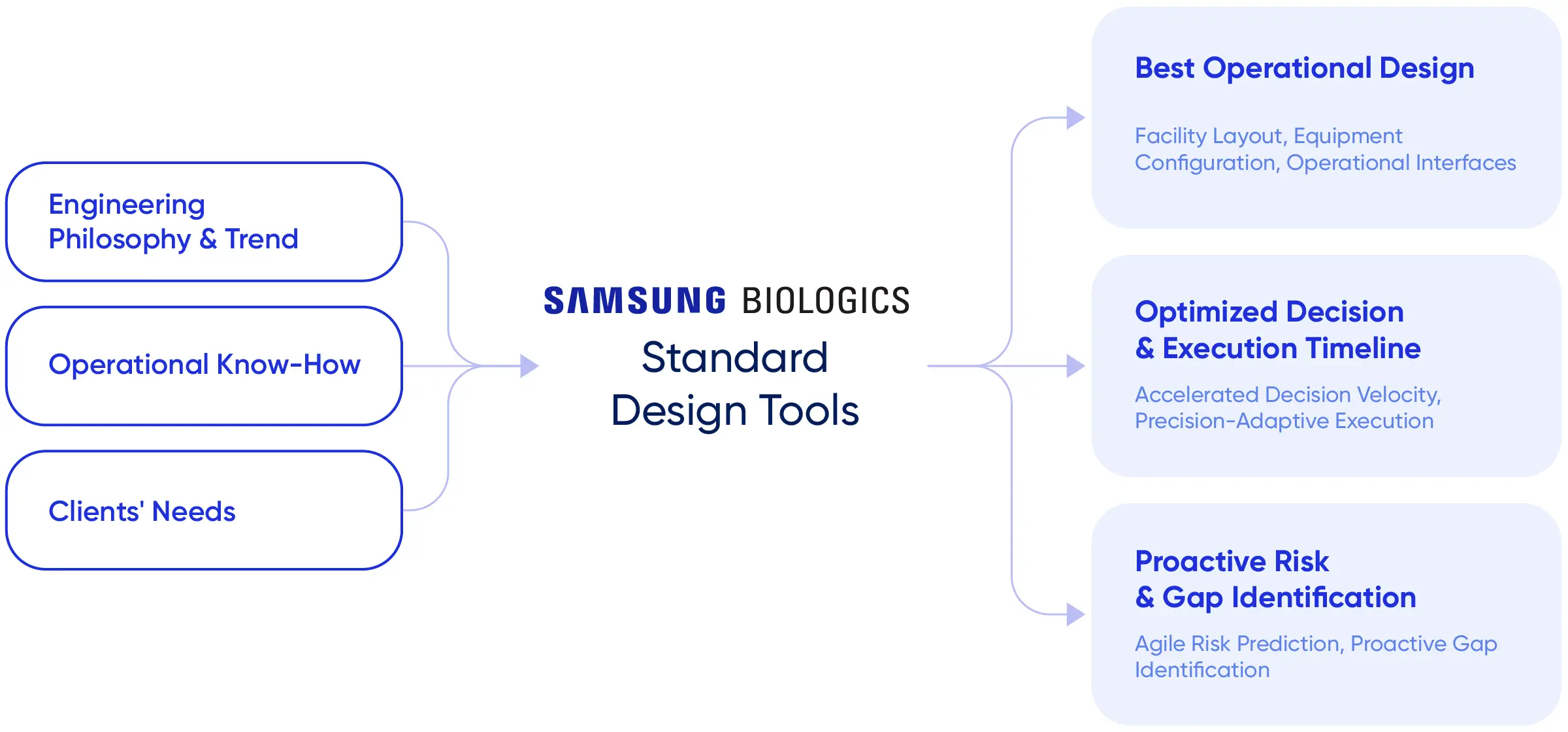
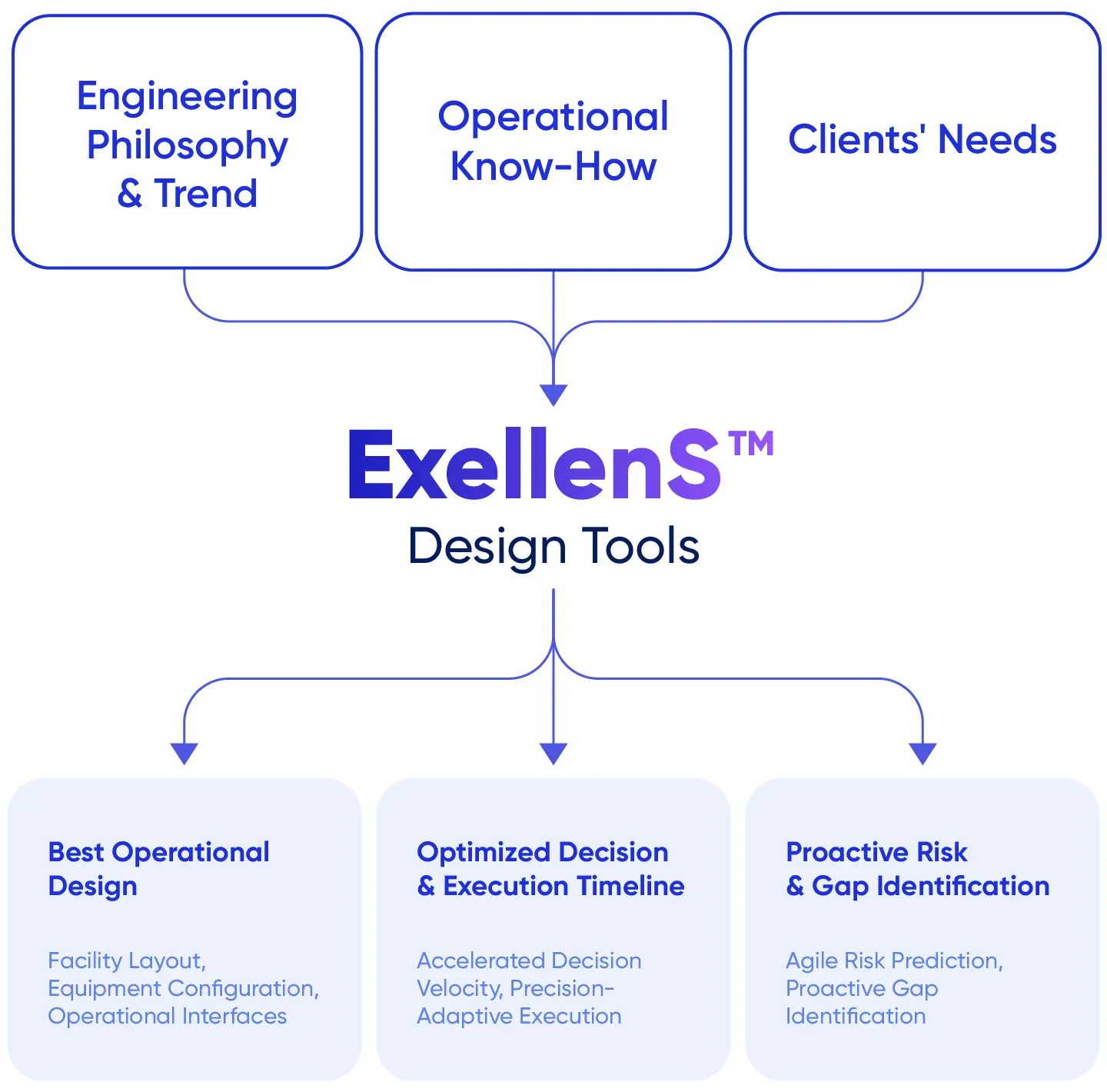
Samsung Biologics has formulated ExellenS™, a standardized manufacturing framework that supports predictable performance. ExellenS™ encompasses accumulated project execution experience and includes automated manufacturability assessment tools and an equivalent facility model. ExellenS™ enables informed manufacturing decisions from project initiation and supports reliable delivery across a global manufacturing network.
Data-driven tool for effective decision-making and project planning
Decisions made during the planning stage can shape manufacturing outcomes long before production begins. To reduce the need for further adjustments and decrease execution risk, it is vital to assess process requirements, facility capabilities, and operational readiness during the planning stages. To support this process, Samsung Biologics developed a suite of data-driven decision support tools that evaluate how and where a given product can be manufactured most effectively across its sites.
At the early planning stage, Samsung Biologics applies mass balance–based modeling tools to assess facility feasibility based on initial project parameters. These tools evaluate manufacturing suitability and identify potential facility gaps by applying data accumulated through prior project execution. This approach supports objective and consistent feasibility assessments while reducing reliance on theoretical assumptions.
For more comprehensive evaluations, such as new facility projects or complex process requirements, the same framework is used to calculate optimal equipment size and configuration based on key variables such as titer, cadence, drug substance characteristics, and process design. The system supports both batch and continuous processes and enables detailed mass balance simulations grounded in extensive manufacturing data.
“Using these tools, teams can rapidly estimate potential manufacturing scenarios across different sites and modalities,” said Jungeun Yang, Engineer in the Corporate Engineering Team. “It supports early-stage decision-making by allowing manufacturing options to be evaluated using execution data and aligned with real operations.”
Establishing a unified model for plant-to-plant consistency
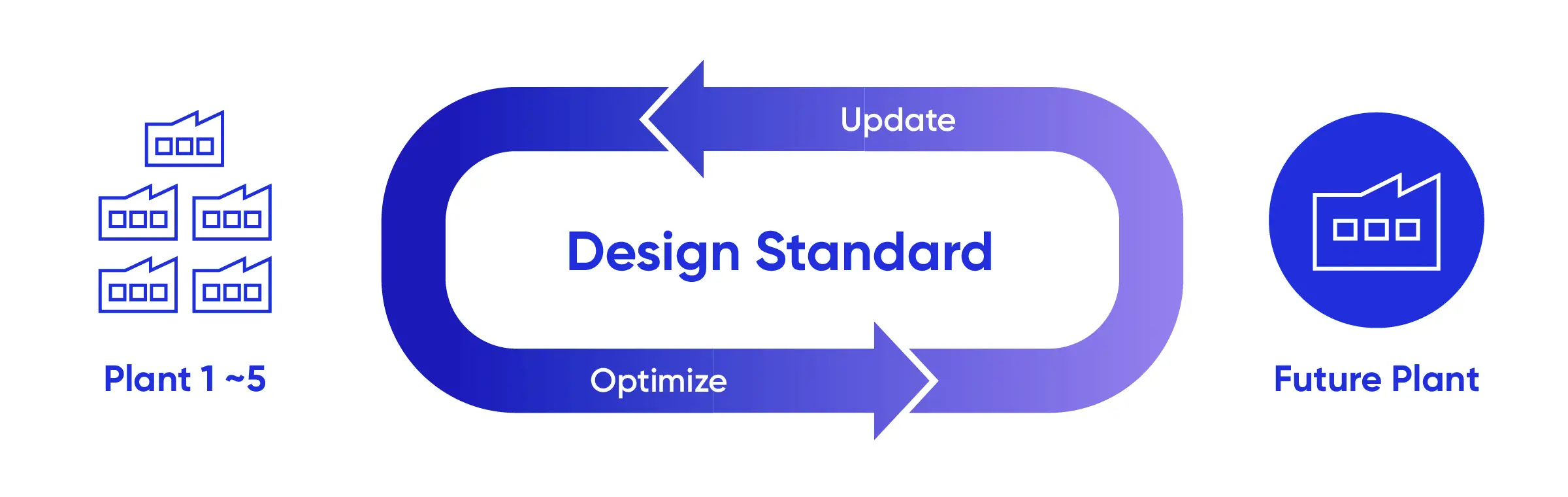
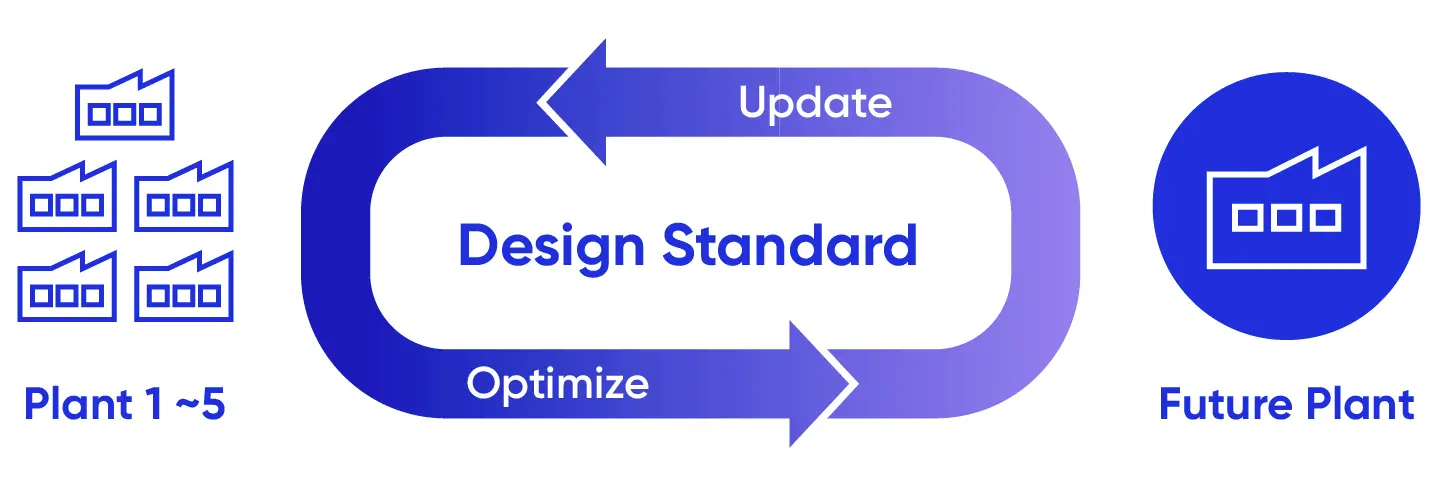
A standardized manufacturing facility design that enables consistent application of manufacturing strategies across sites is central to this approach. Samsung Biologics uses common engineering principles, layouts, and operating concepts to design and operate its facilities, decreasing variability in manufacturing plan execution. This standardization also supports smoother technology transfer, more predictable scale-up, and consistent operational performance.
Alignment across sites relies on systematically reviewing insights from individual projects and sites, rather than managing facilities as independent operations. Applying the insights from these reviews is integral to standardization.
“Standardization provides a stable operational baseline,” Yang explained. “It allows teams to focus on project-specific requirements without introducing unnecessary variability into facility operations.”
Continuous advancement through regular assessment
To ensure that facilities evolve based on how they are used in practice rather than remaining fixed in initial design assumptions, Samsung Biologics continues to refine this framework by regularly assessing project outcomes. Execution learnings, including improvements related to facility layout, equipment configuration, and operational interfaces, are systematically reviewed and incorporated into facility design standards and planning logic.
“Engineering equivalency allows execution experience to be translated into tangible improvements across sites,” Yang noted. “It supports consistency while allowing facilities to evolve as operational requirements change. At Samsung Biologics, we continue to optimize the facility design to further enhance manufacturing reliability and consistency.”
Accommodating project-specific requirements within a standardized model
While consistency requires standardization, manufacturing programs often depend on tailored approaches. Such requirements may vary based on molecule characteristics, development stage, or process complexity. Samsung Biologics’ manufacturing framework can accommodate these differences within its standardized operating model.
The manufacturability assessment tool evaluates project-specific requirements alongside standardized facility capabilities. This assessment supports flexible execution by optimizing manufacturing strategies for each project while using established execution standards.
“Our approach is to standardize the fundamentals while optimizing where complexities exist,” Yang said. “This allows us to adapt to different molecule types and project needs without compromising execution consistency.”
Engineering equivalency supports consistent execution across global sites
This standardized approach establishes a unified operating model across a global network, sets shared expectations, and maintains consistent performance across sites. By applying common engineering standards, data-driven planning methodologies, and execution principles, Samsung Biologics supports reliable delivery across locations, addressing common challenges associated with multi-site manufacturing and reinforcing the predictability of project outcomes.

Predictability remains vital as biopharmaceutical programs become more complex. Samsung Biologics’ standardized manufacturing framework—supported by execution-based data, planning tools, and engineering equivalency—aligns early-stage decisions with enhanced manufacturing readiness.
The company is continuously refining its systems based on project experience to strengthen its ability to support consistent delivery, from project initiation through commercial manufacturing, across its global network.
Early-stage decisions are playing an increasingly important role in the execution success of biopharmaceutical programs. Facility alignment, process fit, and equipment planning considerations can significantly influence timelines, scalability, and long-term supply continuity. As a result, structured manufacturing frameworks that support consistent outcomes across the manufacturing lifecycle are essential for reliable execution.
Samsung Biologics has formulated ExellenS™, a standardized manufacturing framework that supports predictable performance. ExellenS™ encompasses accumulated project execution experience and includes automated manufacturability assessment tools and an equivalent facility model. ExellenS™ enables informed manufacturing decisions from project initiation and supports reliable delivery across a global manufacturing network.
Data-driven tool for effective decision-making and project planning
Decisions made during the planning stage can shape manufacturing outcomes long before production begins. To reduce the need for further adjustments and decrease execution risk, it is vital to assess process requirements, facility capabilities, and operational readiness during the planning stages. To support this process, Samsung Biologics developed a suite of data-driven decision support tools that evaluate how and where a given product can be manufactured most effectively across its sites.
At the early planning stage, Samsung Biologics applies mass balance–based modeling tools to assess facility feasibility based on initial project parameters. These tools evaluate manufacturing suitability and identify potential facility gaps by applying data accumulated through prior project execution. This approach supports objective and consistent feasibility assessments while reducing reliance on theoretical assumptions.
For more comprehensive evaluations, such as new facility projects or complex process requirements, the same framework is used to calculate optimal equipment size and configuration based on key variables such as titer, cadence, drug substance characteristics, and process design. The system supports both batch and continuous processes and enables detailed mass balance simulations grounded in extensive manufacturing data.
“Using these tools, teams can rapidly estimate potential manufacturing scenarios across different sites and modalities,” said Jungeun Yang, Engineer in the Corporate Engineering Team. “It supports early-stage decision-making by allowing manufacturing options to be evaluated using execution data and aligned with real operations.”
Establishing a unified model for plant-to-plant consistency
A standardized manufacturing facility design that enables consistent application of manufacturing strategies across sites is central to this approach. Samsung Biologics uses common engineering principles, layouts, and operating concepts to design and operate its facilities, decreasing variability in manufacturing plan execution. This standardization also supports smoother technology transfer, more predictable scale-up, and consistent operational performance.
Alignment across sites relies on systematically reviewing insights from individual projects and sites, rather than managing facilities as independent operations. Applying the insights from these reviews is integral to standardization.
“Standardization provides a stable operational baseline,” Yang explained. “It allows teams to focus on project-specific requirements without introducing unnecessary variability into facility operations.”
Continuous advancement through regular assessment
To ensure that facilities evolve based on how they are used in practice rather than remaining fixed in initial design assumptions, Samsung Biologics continues to refine this framework by regularly assessing project outcomes. Execution learnings, including improvements related to facility layout, equipment configuration, and operational interfaces, are systematically reviewed and incorporated into facility design standards and planning logic.
“Engineering equivalency allows execution experience to be translated into tangible improvements across sites,” Yang noted. “It supports consistency while allowing facilities to evolve as operational requirements change. At Samsung Biologics, we continue to optimize the facility design to further enhance manufacturing reliability and consistency.”
Accommodating project-specific requirements within a standardized model
While consistency requires standardization, manufacturing programs often depend on tailored approaches. Such requirements may vary based on molecule characteristics, development stage, or process complexity. Samsung Biologics’ manufacturing framework can accommodate these differences within its standardized operating model.
The manufacturability assessment tool evaluates project-specific requirements alongside standardized facility capabilities. This assessment supports flexible execution by optimizing manufacturing strategies for each project while using established execution standards.
“Our approach is to standardize the fundamentals while optimizing where complexities exist,” Yang said. “This allows us to adapt to different molecule types and project needs without compromising execution consistency.”
Engineering equivalency supports consistent execution across global sites
This standardized approach establishes a unified operating model across a global network, sets shared expectations, and maintains consistent performance across sites. By applying common engineering standards, data-driven planning methodologies, and execution principles, Samsung Biologics supports reliable delivery across locations, addressing common challenges associated with multi-site manufacturing and reinforcing the predictability of project outcomes.
Predictability remains vital as biopharmaceutical programs become more complex. Samsung Biologics’ standardized manufacturing framework—supported by execution-based data, planning tools, and engineering equivalency—aligns early-stage decisions with enhanced manufacturing readiness.
The company is continuously refining its systems based on project experience to strengthen its ability to support consistent delivery, from project initiation through commercial manufacturing, across its global network.
Share article
Related Content