Exploring Samsung Biologics’ CDO capabilities | Bispecific antibodies

Driven by advances in biotechnology, biopharmaceuticals are among the most sophisticated achievements of modern science and have become central to the industry. Antibody therapies, making up the largest share, have dramatically evolved since the approval of the first monoclonal antibody by the U.S. Food and Drug Administration in 1986.
The efficacy and safety of bispecific antibodies, combined with their ability to address previously untreatable conditions and multifaceted diseases, illustrates how they represent a key component of the next-generation of antibody therapy.
According to the research and consulting firm Roots Analysis, the global bispecific antibody market is expected to grow at an annual rate of 34% to top $9 billion by 2030. The market is considered a blue ocean, with only two bispecific antibodies approved by both the FDA and European Medicine Agency for the treatment of cancer patients.
Attractive treatment modality
Bispecific antibodies are capable of targeting two distinct antigens simultaneously, improving treatment potential compared to the conventional monoclonal antibodies that only target one type of antigen.
Many complex diseases are driven by a variety of factors, which can circumvent the impact of a drug. Bispecific antibodies can improve the specificity and effectiveness, such as in the case of cancer immunotherapy. One arm binds to an antigen on a cancer cell, while the other binds to T-cells, bringing both cell types together to activate T-cells to eliminate the cancer cells.
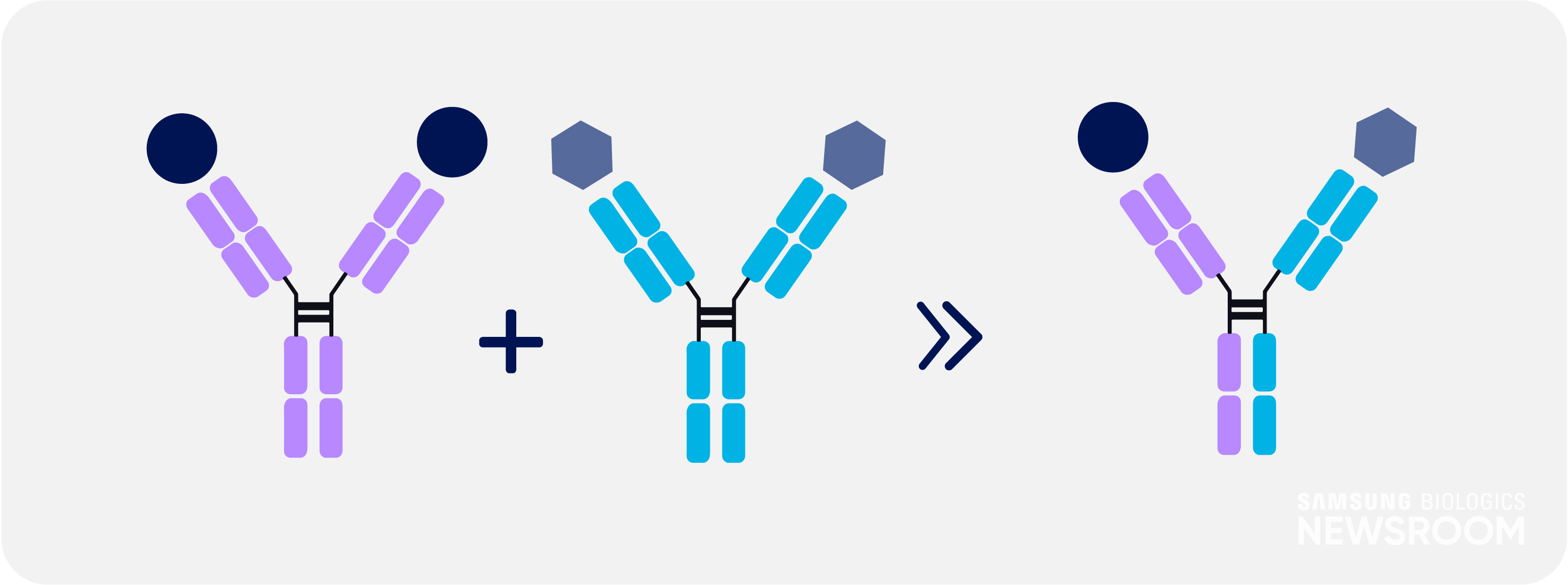
However, there have been challenges in development of bispecific antibodies, especially regarding immunogenicity and chain mispairing, while issues in respect to the quality and stability have hampered their wider clinical application.
S-DUALTM boasts safety and optimal manufacturability
Samsung Biologics launched a new proprietary development technology platform, S-DUAL™ in September, boosting its strength in the CDO service. Having a unique asymmetric design, S-DUAL™ has high purity, yield and productivity.

In the production of bispecific antibodies, only one of eight possible combinations is bispecific – requiring advanced technology to differentiate it. With a unique asymmetric design, S-DUAL™ can effectively separate and analyze the bispecific antibody as impurities from the improper pairings form different sizes.
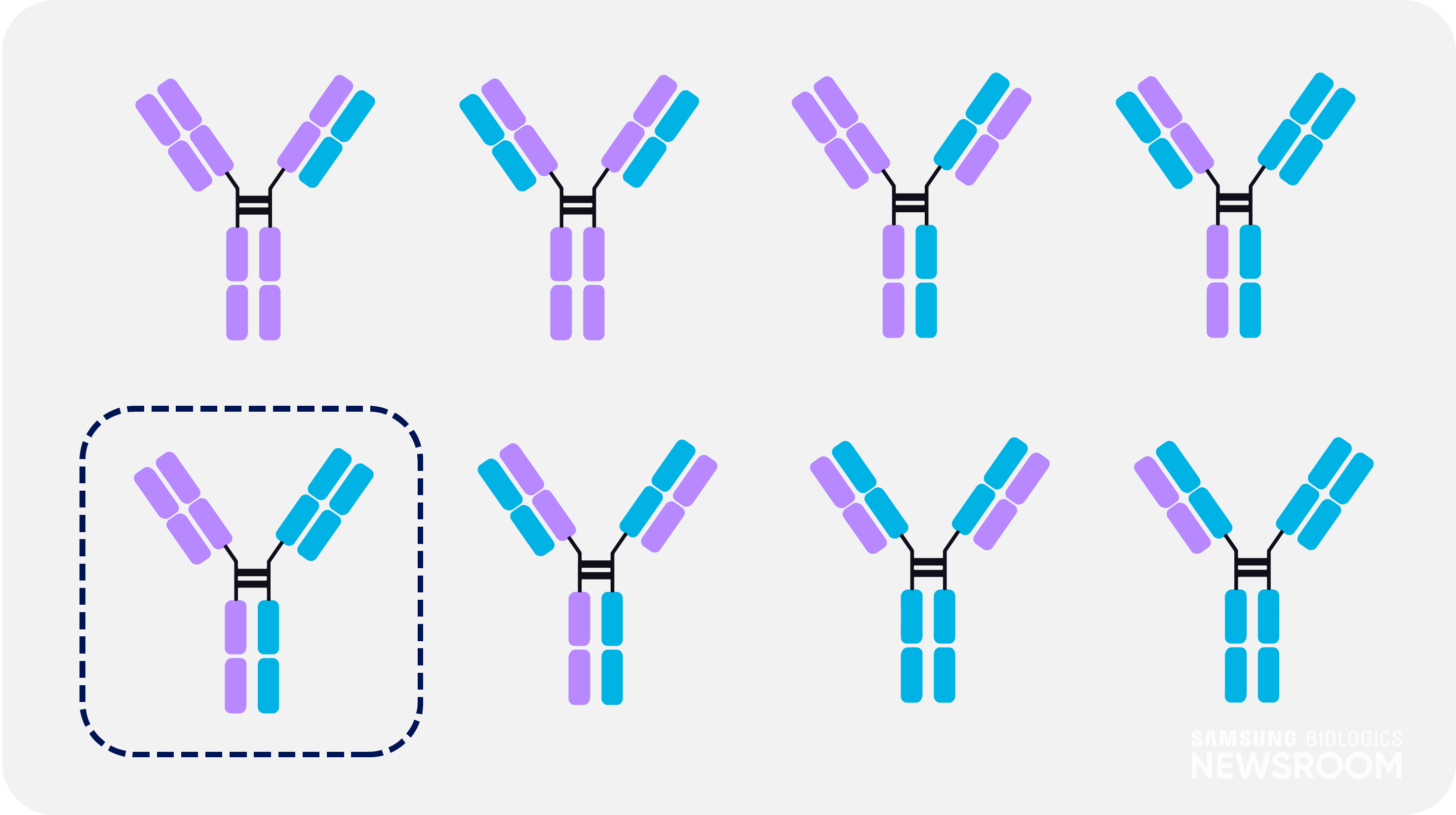
It also applies ‘knob-in-hole’ technique to avoid mispairing between two heavy chains and light chains. This strategy provides high yield and maximum purity of 99%.
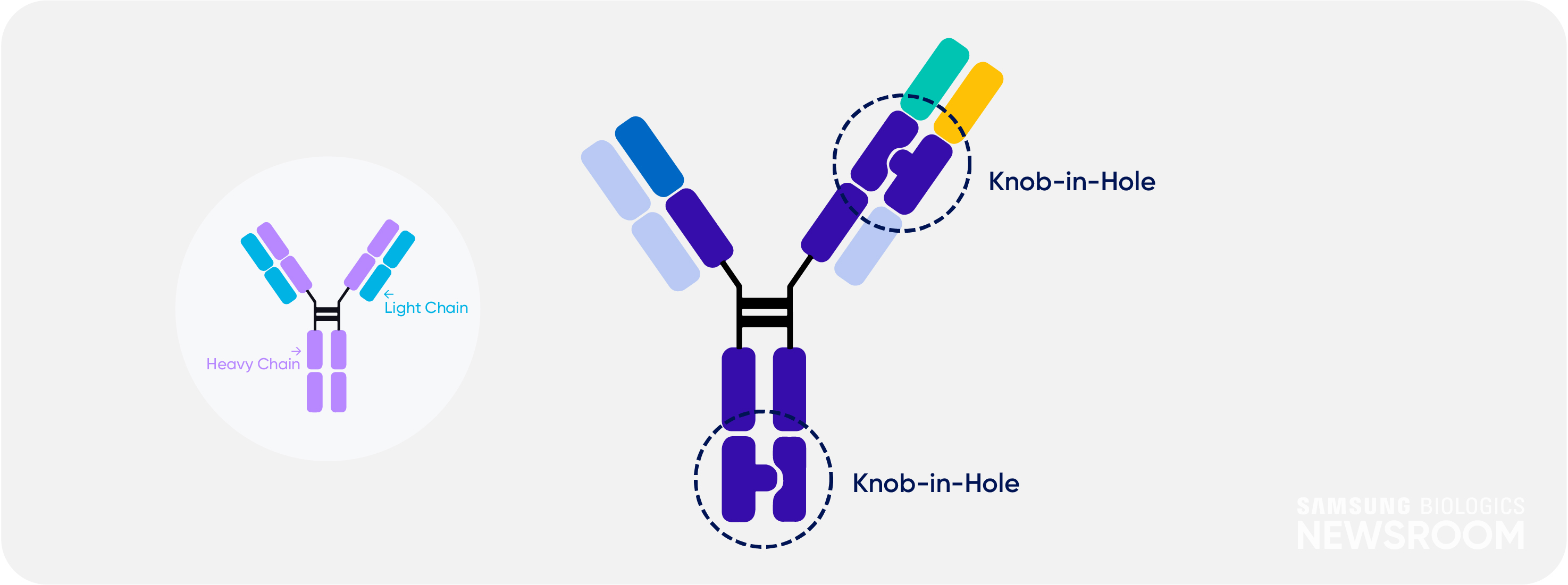
S-DUAL™ also has low immunogenicity due to human immunoglobulin gamma (lgG)-like structure. Samsung Biologics can design tailored antibody structures depending on clients’ needs.
Biopharma’s new drug development partner
As Samsung Biologics provides end-to-end services, from contract development to contract manufacturing, it has a competitive edge in new drug development, where speed is crucial. Samsung Biologics has received over 160 regulatory approvals as of October 2022.
The company has also strengthened its foothold as a new drug development partner by establishing a one-stop service from cell line development to commercial manufacturing, and opened an CDO R&D Center in San Francisco to provide services in close proximity to clients.
To meet fast-changing industry trends, Samsung also launched DEVELOPICK™, a developability assessment platform that screens molecules at an early stage to identify candidates with the best potential for advancement to Investigational New Drug, alongside the S-DUAL™. Samsung Biologics will continue to invest and take action to lead in the next-generation of biopharmaceuticals.

Driven by advances in biotechnology, biopharmaceuticals are among the most sophisticated achievements of modern science and have become central to the industry. Antibody therapies, making up the largest share, have dramatically evolved since the approval of the first monoclonal antibody by the U.S. Food and Drug Administration in 1986.
The efficacy and safety of bispecific antibodies, combined with their ability to address previously untreatable conditions and multifaceted diseases, illustrates how they represent a key component of the next-generation of antibody therapy.
According to the research and consulting firm Roots Analysis, the global bispecific antibody market is expected to grow at an annual rate of 34% to top $9 billion by 2030. The market is considered a blue ocean, with only two bispecific antibodies approved by both the FDA and European Medicine Agency for the treatment of cancer patients.
Attractive treatment modality
Bispecific antibodies are capable of targeting two distinct antigens simultaneously, improving treatment potential compared to the conventional monoclonal antibodies that only target one type of antigen.
Many complex diseases are driven by a variety of factors, which can circumvent the impact of a drug. Bispecific antibodies can improve the specificity and effectiveness, such as in the case of cancer immunotherapy. One arm binds to an antigen on a cancer cell, while the other binds to T-cells, bringing both cell types together to activate T-cells to eliminate the cancer cells.

However, there have been challenges in development of bispecific antibodies, especially regarding immunogenicity and chain mispairing, while issues in respect to the quality and stability have hampered their wider clinical application.
S-DUALTM boasts safety and optimal manufacturability
Samsung Biologics launched a new proprietary development technology platform, S-DUAL™ in September, boosting its strength in the CDO service. Having a unique asymmetric design, S-DUAL™ has high purity, yield and productivity.

In the production of bispecific antibodies, only one of eight possible combinations is bispecific – requiring advanced technology to differentiate it. With a unique asymmetric design, S-DUAL™ can effectively separate and analyze the bispecific antibody as impurities from the improper pairings form different sizes.

It also applies ‘knob-in-hole’ technique to avoid mispairing between two heavy chains and light chains. This strategy provides high yield and maximum purity of 99%.

S-DUAL™ also has low immunogenicity due to human immunoglobulin gamma (lgG)-like structure. Samsung Biologics can design tailored antibody structures depending on clients’ needs.
Biopharma’s new drug development partner
As Samsung Biologics provides end-to-end services, from contract development to contract manufacturing, it has a competitive edge in new drug development, where speed is crucial. Samsung Biologics has received over 160 regulatory approvals as of October 2022.
The company has also strengthened its foothold as a new drug development partner by establishing a one-stop service from cell line development to commercial manufacturing, and opened an CDO R&D Center in San Francisco to provide services in close proximity to clients.

To meet fast-changing industry trends, Samsung also launched DEVELOPICK™, a developability assessment platform that screens molecules at an early stage to identify candidates with the best potential for advancement to Investigational New Drug, alongside the S-DUAL™. Samsung Biologics will continue to invest and take action to lead in the next-generation of biopharmaceuticals.
Share article
Related Content