Overview
-
Empowering
Your Biologics
Manufacturing
Journey -
Samsung Biologics is a reliable partner for seamless tech transfers, clinical and commercial biologics manufacturing, aseptic fill-finish and analytical testing. From the beginning of our partnership, you will have a dedicated project management team to walk you through the entire product development and manufacturing journey. We are committed to excellence in CGMP biologics manufacturing and quality assurance to deliver value to our clients.
Values for Customer Satisfaction in Biologics Manufacturing
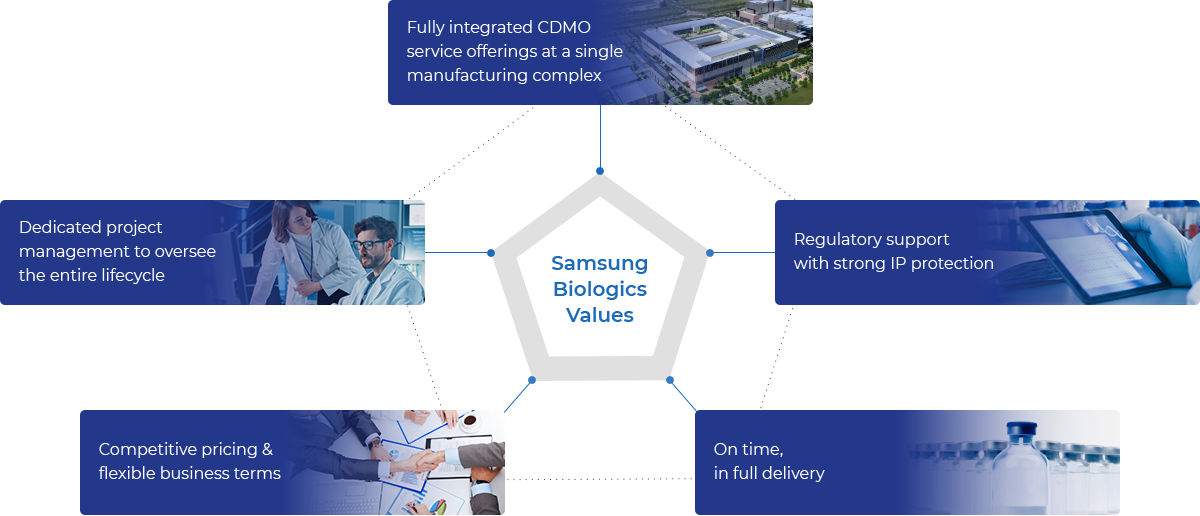
Samsung Biologics values customer satisfaction in biologics manufacturing with a fully integrated CDMO service for CGMP biologics manufacturing.
-
Fully integrated CDMO
service offerings at a single
manufacturing complex -
Dedicated project
managerment to oversee
the entire lifecycle -
Regulatory support
with strong IP protection -
Competitive pricing &
flexible business terms -
On time,
in full delivery
Biologics Contract Manufacturing Capabilities
-
Early Clinical
Production -
Early clinical material production involves risks and uncertainties. We are committed to providing precisely tailored solutions to mitigate the risk with our strong drug development capabilities and flexible capacities.
-
CGMP Commercial
Production -
Rising competition requires biological drug developeras to take a systematic, cost-effective approach to manufacturing. Samsung Biologics achieves fast turnaround times with an economy of scale to quickly deliver high-quality products to market.
- Aseptic Fill-Finish
-
Aseptic fill finish is critical in drug manufacturing to ensure patient and product safety. Samsung Biologics provides clinical and commercial fill-finish services to provide the highest possible quality.
Connect with our manufacturing experts