
As one of the most critical steps in biomanufacturing, the drug product process requires scrupulous control and maintenance, attention to quality and extensive expertise for a successful clinical or commercial launch. Companies that do not have a reliable process in place could face challenges, costing time and money.
At Samsung Biologics, we employ state-of-the-art technologies to make and deliver flawless drug products to our clients. Below is an in-depth look at how we manufacture these products at our headquarters in Songdo, South Korea.
What is a Drug Product?
Biomedicine is categorized into two elements: Drug Substance (DS) and Drug Product (DP). Drug substance, an ingredient used for producing DPs, is a material generated via either synthesis, fermentation or extraction, or a combination of these three methods. For instance, extracted antibodies through cell culture and purification processes are considered drug substances. These substances then get transformed into a dosage form called drug product — which, after being aseptically filled into either a vial, cartridge or syringe, gets safely injected into a patient’s body.
How is a Drug Product Manufactured?
Samsung Biologics manufactures drug products by using drug substances manufactured at its own site or delivered from clients.

Drug Product Manufacturing Stages
- > Formulation: First, scientists will integrate the characterization of client molecule with the final product format, developing a formulation that will ensure the delivery of a stable product. Depending on characteristics of substances, buffers are utilized in this stage to maintain safety.
- > Aseptic Filling: Once formulated, substances – in either a liquid or lyophilized form – get filled into a container such as a vial, pre-filled syringe or cartridge. If needed, a filling conducted in a lyophilized form adds maximum stability to biomedicines as lyophilization eliminates any remaining liquid or moisture inside the container.
- > Inspection: After being filled, biomedicines undergo a series of inspections to confirm they meet compliance and thus are safe for bodily injection.
- > Labeling and Packaging: And finally, drug products get labeled and packaged for shipment.
Client-Optimized, End-to-End Solution
Samsung Biologics provides a one-stop, end-to-end solution that consists of aseptic filling, visual inspection, labeling and packaging, release testing and regulatory support. To ensure flawless quality of drug products manufactured and delivered to clients across continents, Samsung Biologics may utilize stainless steel or a single-use system not only to accommodate distinctive manufacturing preferences from clients but also to ensure the quality of the drug product.
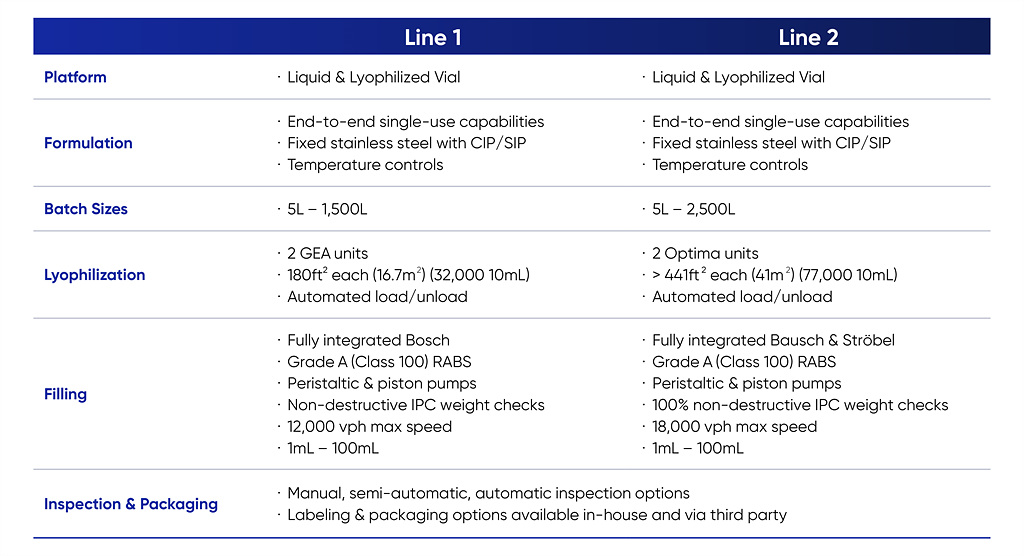 Samsung Biologics’ Fill-Finish manufacturing capabilities
Samsung Biologics’ Fill-Finish manufacturing capabilities
The company has for years managed to meet the needs of its biopharma clients, both big and small, as it not only operates batches sized from 5L to 2,500L but also employs a small-scale clinical Flexible Filling Line (FFL). With the world’s largest CMO capacity, Samsung Biologics’ fully integrated drug substance and drug product manufacturing can help clients save operational time and cost.
Additionally, the company employs a dedicated, expert MSAT team to efficiently execute optimization of process technology from the tech transfer stage. Furthermore, a thorough maintenance at the manufacturing stage is conducted to produce high-quality biomedicines as well as regulatory support in the subsequent stages for the sake of clients.
Drug products must be rigorously inspected for their safety and effectiveness as they get directly injected into patients. Samsung Biologics has earned more than 63 drug product manufacturing approvals from global regulatory agencies including the U.S. Food and Drug Administration (FDA), the European Medicine Agency (EMA) and Japan’s Pharmaceuticals and Medical Devices Agency.
Preparing for a Healthier Future with Samsung Biologics
Drug substances and products make up about 57% and 43%, respectively, of the total share of the global biomedicine contract manufacturing, according to a market research report by Frost & Sullivan. The drug product market will likely expand as the global biomedicine industry continues to grow.

As demand for injectable drug products is forecasted to increase, Samsung Biologics has for years delivered quality biomedicines to patients across the globe with the help of its extensive biomanufacturing capacity and seamless supply chain system. Through its commitment to achieving “Better Life” for humanity through biomedicines, Samsung Biologics will continue to build a healthier future for the world.

As one of the most critical steps in biomanufacturing, the drug product process requires scrupulous control and maintenance, attention to quality and extensive expertise for a successful clinical or commercial launch. Companies that do not have a reliable process in place could face challenges, costing time and money.
At Samsung Biologics, we employ state-of-the-art technologies to make and deliver flawless drug products to our clients. Below is an in-depth look at how we manufacture these products at our headquarters in Songdo, South Korea.
What is a Drug Product?
Biomedicine is categorized into two elements: Drug Substance (DS) and Drug Product (DP). Drug substance, an ingredient used for producing DPs, is a material generated via either synthesis, fermentation or extraction, or a combination of these three methods. For instance, extracted antibodies through cell culture and purification processes are considered drug substances. These substances then get transformed into a dosage form called drug product — which, after being aseptically filled into either a vial, cartridge or syringe, gets safely injected into a patient’s body.
How is a Drug Product Manufactured?
Samsung Biologics manufactures drug products by using drug substances manufactured at its own site or delivered from clients.

Drug Product Manufacturing Stages
- > Formulation: First, scientists will integrate the characterization of client molecule with the final product format, developing a formulation that will ensure the delivery of a stable product. Depending on characteristics of substances, buffers are utilized in this stage to maintain safety.
- > Aseptic Filling: Once formulated, substances – in either a liquid or lyophilized form – get filled into a container such as a vial, pre-filled syringe or cartridge. If needed, a filling conducted in a lyophilized form adds maximum stability to biomedicines as lyophilization eliminates any remaining liquid or moisture inside the container.
- > Inspection: After being filled, biomedicines undergo a series of inspections to confirm they meet compliance and thus are safe for bodily injection.
- > Labeling and Packaging: And finally, drug products get labeled and packaged for shipment.
Client-Optimized, End-to-End Solution
Samsung Biologics provides a one-stop, end-to-end solution that consists of aseptic filling, visual inspection, labeling and packaging, release testing and regulatory support. To ensure flawless quality of drug products manufactured and delivered to clients across continents, Samsung Biologics may utilize stainless steel or a single-use system not only to accommodate distinctive manufacturing preferences from clients but also to ensure the quality of the drug product.
 Samsung Biologics’ Fill-Finish manufacturing capabilities
Samsung Biologics’ Fill-Finish manufacturing capabilities
The company has for years managed to meet the needs of its biopharma clients, both big and small, as it not only operates batches sized from 5L to 2,500L but also employs a small-scale clinical Flexible Filling Line (FFL). With the world’s largest CMO capacity, Samsung Biologics’ fully integrated drug substance and drug product manufacturing can help clients save operational time and cost.
Additionally, the company employs a dedicated, expert MSAT team to efficiently execute optimization of process technology from the tech transfer stage. Furthermore, a thorough maintenance at the manufacturing stage is conducted to produce high-quality biomedicines as well as regulatory support in the subsequent stages for the sake of clients.
Drug products must be rigorously inspected for their safety and effectiveness as they get directly injected into patients. Samsung Biologics has earned more than 63 drug product manufacturing approvals from global regulatory agencies including the U.S. Food and Drug Administration (FDA), the European Medicine Agency (EMA) and Japan’s Pharmaceuticals and Medical Devices Agency.
Preparing for a Healthier Future with Samsung Biologics
Drug substances and products make up about 57% and 43%, respectively, of the total share of the global biomedicine contract manufacturing, according to a market research report by Frost & Sullivan. The drug product market will likely expand as the global biomedicine industry continues to grow.

As demand for injectable drug products is forecasted to increase, Samsung Biologics has for years delivered quality biomedicines to patients across the globe with the help of its extensive biomanufacturing capacity and seamless supply chain system. Through its commitment to achieving “Better Life” for humanity through biomedicines, Samsung Biologics will continue to build a healthier future for the world.
Share article
Related Content