A robust cell line is a must-have for raw molecules to become a full-fledged drug. Without generating a high-titer cell line, biotech companies would have to risk facing the low commercial viability of their drug-development project from the beginning. In a continuous effort to help de-risk and maximize the project's commerciality, Samsung Biologics has enhanced its early drug-development platforms, a foundation for one-stop drug manufacturing.
Read how a globally leading contract development and manufacturing organization (CDMO) is prepared to advance its clients’ molecules to the Investigational New Drug stage and beyond with maximized quality and minimized risk.
Whether to successfully advance a molecule from late discovery to commercialization depends on how many risks can be identified and addressed at an assessment stage. Risks may stem from not having fully implemented a molecule’s characterization and failing to sort out low-potential drug candidates, Samsung Biologics has recently launched a late drug-discovery platform, S-CHOsient™, to phase out such risks.
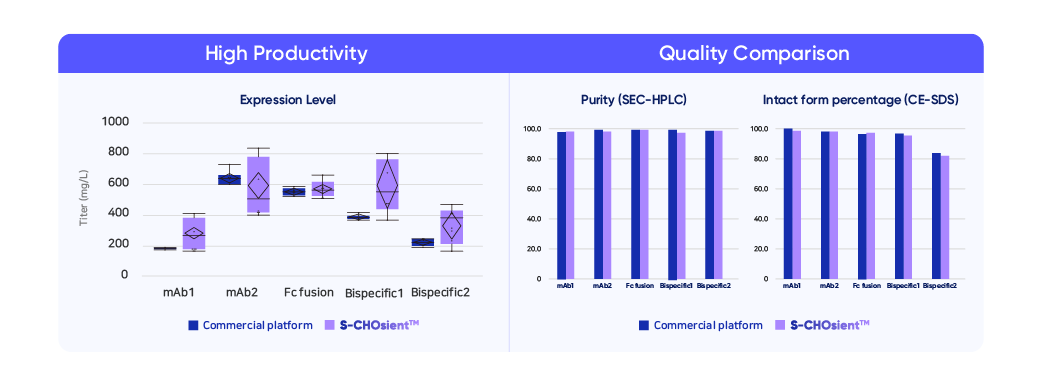
By transiently expressing high-quality genes at different scales, S-CHOsient™ increases the reliability and performance of the developability assessment platform, DEVELOPICK™. Testing across various modalities, from monoclonal antibodies to Fc-fusion proteins, has yielded a higher titer under a condensed timeline when compared to results from other industry platforms. Quality comparisons, based on percent purity analysis, demonstrate compatibility, enabling a wide range of options for our clients' development needs. “We wanted to add S-CHOsient™ to our development platform portfolio to help build a reliable foundation for successful drug development for our clients,” said Lee, Director of Cell Line Development at Samsung Biologics. “By assessing a thorough molecule characterization and expressing robust genes, the platform prepares our clients to unleash the full potential of their molecules.”
Navigating the intricate pathway to biopharmaceutical commercialization also demands careful selection of the right sequence or molecule for development. Samsung addresses this challenge through DEVELOPICK™, a tool that assesses the physical-chemical properties of molecules, providing crucial insights into purification processes and setting the stage for efficient cell line development (CLD).
Developing strong cell lines through an enhanced CLD platform
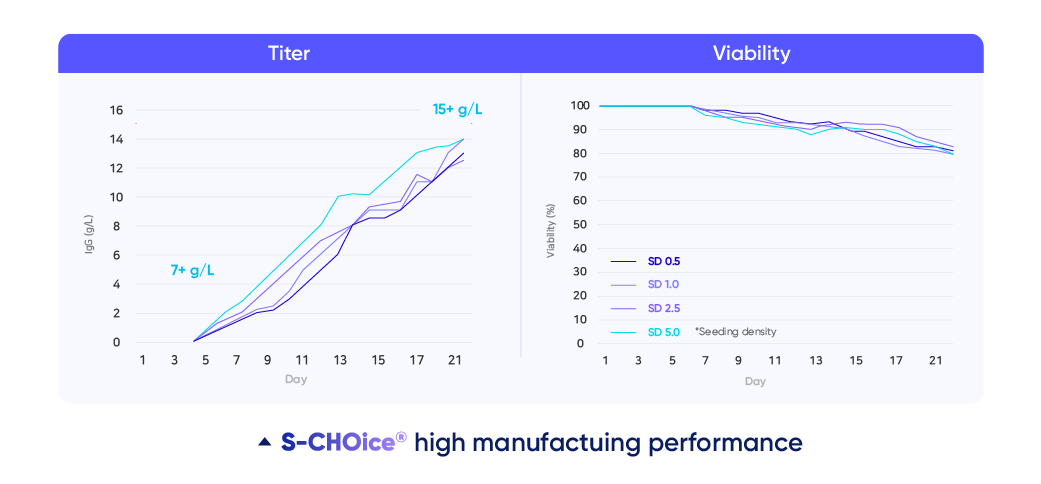
Samsung Biologics’ CLD platform is rigorously designed to generate robust cell lines. S-CHOice®, a high-performing proprietary cell line platform built on glutamine synthase (GS) knock-out Chinese hamster ovary (CHO) cell line technology, shows improved titers up to two-fold from industry average, reaching above 7g/L for standard monoclonal antibodies. The cell line also shows enhanced cell viability with over 90% at day-21 in a fed-batch study, demonstrating effectiveness in producing high-quality cell lines. With both standard and transposase processes, the platform has successfully developed over 60% of monoclonal and 20% of bispecific antibodies.
The recent introduction of a transposase gene integration system further enhances titers. With the capability for multiple insertions across the genome and applicability to genes of all sizes, while maintaining structural integrity, the transposase system has resulted in notable increases in productivity, purity, and stability. S-CHOice® generated a 3 to 7-fold increase in cell-line productivity and a significant 33% reduction in the standard timeline for monoclonal antibodies, leading to a two-month period from transfection to Research Cell Bank (RCB)* selection. Additionally, bispecific antibodies experienced a 25% reduction, allowing for a three-month period from transfection to RCB selection.
Fostering End-to-End Collaboration for Challenging Case
Seamless communication and collaboration across teams at a single site is another key advantage of partnering with Samsung Biologics.
In a recent partnership, Samsung Biologics addressed a client's low-expressing CHO cell line (IgG4) challenge through its S-CHOice® CLD platform, achieving a notable 2.4-fold increase in ex-pression. However, during quality analysis slight deviations were revealed in critical quality attributes (CQA), including the charge variants. The analytical team promptly responded, strategically leveraging process parameters and component lists to address the quality aspects.
This collaborative effort extended to the upstream and downstream teams, that identified critical quality attributes and had a feasibility run, allowing the team to optimize processes for purity, yields, and comparability at the drug substance phase. The successful screening early in the project, guided by the right analytical methods, resulted in a remarkable 3.4-fold overall increase in productivity. This case study underscores the effectiveness of end-to-end collaboration, showcasing the ability to overcome challenges, achieve higher productivity, and meet client expectations.
Continuous platform enhancement driven by client-centric mindset
Client satisfaction is our No. 1 priority in our business operations. This is why we have proactively been investing in upgrading and diversifying our drug-development platforms, to help as many drug developers as possible in advancing their molecules to IND submissions and ultimately to commercialization. Coupled with our competitive platforms, our depth of development knowledge, and experience have brought a track record of 240 successful IND submissions. For more information on our CDO services, please reach out to our experts at Samsungbiologics.com/contact-us.
A robust cell line is a must-have for raw molecules to become a full-fledged drug. Without generating a high-titer cell line, biotech companies would have to risk facing the low commercial viability of their drug-development project from the beginning. In a continuous effort to help de-risk and maximize the project's commerciality, Samsung Biologics has enhanced its early drug-development platforms, a foundation for one-stop drug manufacturing.
Read how a globally leading contract development and manufacturing organization (CDMO) is prepared to advance its clients’ molecules to the Investigational New Drug stage and beyond with maximized quality and minimized risk.

Unleashing the Full Potential of a Molecule
Whether to successfully advance a molecule from late discovery to commercialization depends on how many risks can be identified and addressed at an assessment stage. Risks may stem from not having fully implemented a molecule’s characterization and failing to sort out low-potential drug candidates, Samsung Biologics has recently launched a late drug-discovery platform, S-CHOsient™, to phase out such risks.
By transiently expressing high-quality genes at different scales, S-CHOsient™ increases the reliability and performance of the developability assessment platform, DEVELOPICK™. Testing across various modalities, from monoclonal antibodies to Fc-fusion proteins, has yielded a higher titer under a condensed timeline when compared to results from other industry platforms. Quality comparisons, based on percent purity analysis, demonstrate compatibility, enabling a wide range of options for our clients' development needs. “We wanted to add S-CHOsient™ to our development platform portfolio to help build a reliable foundation for successful drug development for our clients,” said Lee, Director of Cell Line Development at Samsung Biologics. “By assessing a thorough molecule characterization and expressing robust genes, the platform prepares our clients to unleash the full potential of their molecules.”

Navigating the intricate pathway to biopharmaceutical commercialization also demands careful selection of the right sequence or molecule for development. Samsung addresses this challenge through DEVELOPICK™, a tool that assesses the physical-chemical properties of molecules, providing crucial insights into purification processes and setting the stage for efficient cell line development (CLD).
Developing strong cell lines through an enhanced CLD platform
Samsung Biologics’ CLD platform is rigorously designed to generate robust cell lines. S-CHOice®, a high-performing proprietary cell line platform built on glutamine synthase (GS) knock-out Chinese hamster ovary (CHO) cell line technology, shows improved titers up to two-fold from industry average, reaching above 7g/L for standard monoclonal antibodies. The cell line also shows enhanced cell viability with over 90% at day-21 in a fed-batch study, demonstrating effectiveness in producing high-quality cell lines. With both standard and transposase processes, the platform has successfully developed over 60% of monoclonal and 20% of bispecific antibodies.

The recent introduction of a transposase gene integration system further enhances titers. With the capability for multiple insertions across the genome and applicability to genes of all sizes, while maintaining structural integrity, the transposase system has resulted in notable increases in productivity, purity, and stability. S-CHOice® generated a 3 to 7-fold increase in cell-line productivity and a significant 33% reduction in the standard timeline for monoclonal antibodies, leading to a two-month period from transfection to Research Cell Bank (RCB)* selection. Additionally, bispecific antibodies experienced a 25% reduction, allowing for a three-month period from transfection to RCB selection.
Fostering End-to-End Collaboration for Challenging Case
Seamless communication and collaboration across teams at a single site is another key advantage of partnering with Samsung Biologics.
In a recent partnership, Samsung Biologics addressed a client's low-expressing CHO cell line (IgG4) challenge through its S-CHOice® CLD platform, achieving a notable 2.4-fold increase in ex-pression. However, during quality analysis slight deviations were revealed in critical quality attributes (CQA), including the charge variants. The analytical team promptly responded, strategically leveraging process parameters and component lists to address the quality aspects.
This collaborative effort extended to the upstream and downstream teams, that identified critical quality attributes and had a feasibility run, allowing the team to optimize processes for purity, yields, and comparability at the drug substance phase. The successful screening early in the project, guided by the right analytical methods, resulted in a remarkable 3.4-fold overall increase in productivity. This case study underscores the effectiveness of end-to-end collaboration, showcasing the ability to overcome challenges, achieve higher productivity, and meet client expectations.
Continuous platform enhancement driven by client-centric mindset
Client satisfaction is our No. 1 priority in our business operations. This is why we have proactively been investing in upgrading and diversifying our drug-development platforms, to help as many drug developers as possible in advancing their molecules to IND submissions and ultimately to commercialization. Coupled with our competitive platforms, our depth of development knowledge, and experience have brought a track record of 240 successful IND submissions. For more information on our CDO services, please reach out to our experts at Samsungbiologics.com/contact-us .
Share article
Related Content