BioProcess International 2022 | Samsung Biologics unveils new development platforms to offer differentiated services

Samsung Biologics introduced two new development technology platforms at BioProcess International 2022 between September 27-30 in Boston. The conference is the largest bioprocessing event, inviting over 1,800 experts to discuss how to accelerate promising biologics, cell and gene therapies towards commercial success.
Launching platforms S-DUALTM and DEVELOPICKTM
At this year’s event, Samsung Biologics showcased its strength in the CDO business by introducing new proprietary development technology platforms: S-DUAL™ and DEVELOPICK™.





S-DUAL™ is a high-yield bispecific antibody platform with a 99% chain-pairing success rate. Its unique asymmetrical structure reduces the risk of mispairing and ensures high binding affinity among chains to produce high titer and purity for optimized manufacturability. Bispecific antibodies are capable of targeting two different antigens at once, improving treatment potential. According to the research and consulting firm Roots Analysis, the global bispecific antibody market is expected to grow at an annual rate of 34% to reach 9.6 billion USD by 2030.
DEVELOPICK™ is a rapid developability assessment platform that systematically screens molecules at an early stage to identify candidates with the best potential for advancement to Investigational New Drug. The assessment platform enables clients to conduct a robust risk assessment for unsorted drug candidates within a month. By utilizing DEVELOPICK™, Samsung Biologics can provide early insight and selection guidance to save time and cost, and ultimately maximize efficiency.
Promoting Strengths of Differentiated CDO Services and Bolstering Networking

On September 30, Wonjun Yang, Lead Scientist for Antibody-Based Research at Samsung Biologics, shared his insights and new approaches in bispecific antibody development.
Samsung Biologics also held a total of four poster sessions to promote DEVELOPICK™ and its MSAT (Manufacturing Science and Technology) capabilities during the three-day event to showcase the company’s competency in the contract development business.

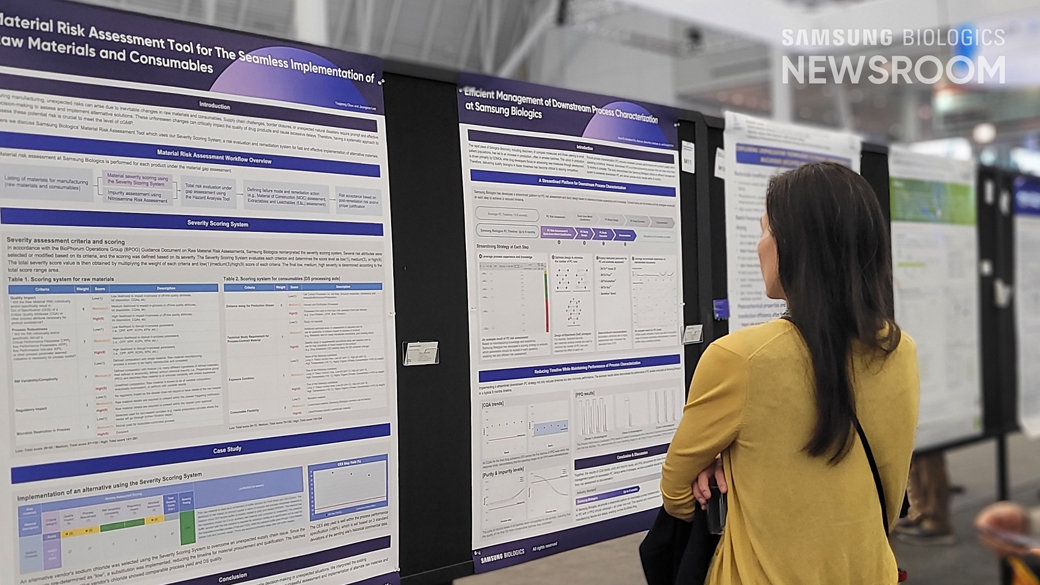
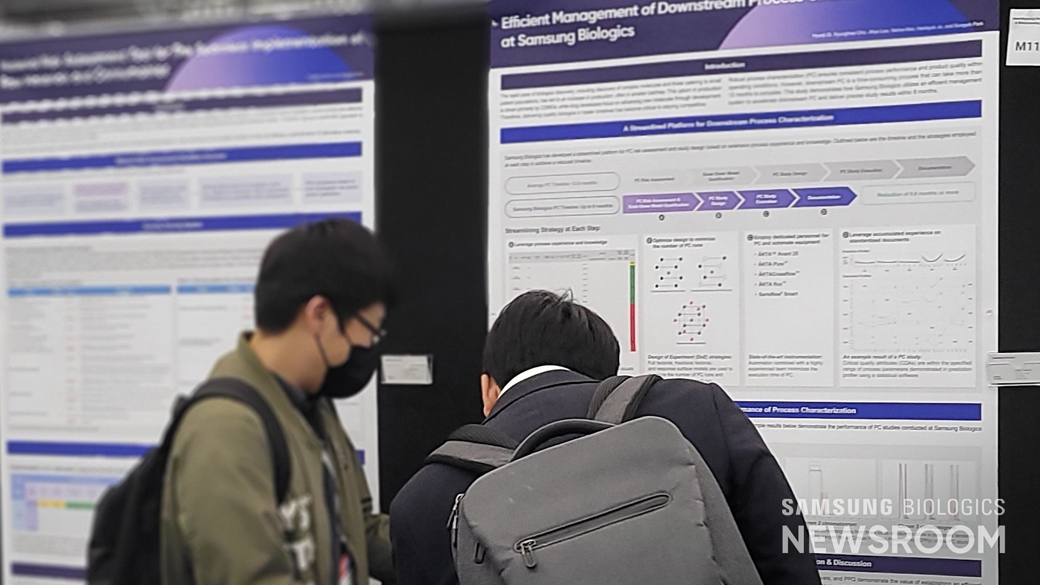
Solidifying Global Foothold as a Partner for Successful Development of New Drugs

Samsung Biologics has won over 100 projects since it first launched its CDO business in 2018. It offers end-to-end, one-stop services, from cell line development to integrated manufacturing from clinical to commercial, enabling cost and time reduction for development.
Samsung Biologics continues to strengthen its foothold in the global contract development market, by opening an R&D Center in San Francisco in 2020, launching its proprietary cell line, S-CHOice® in 2021, and the ‘S-DUALTM’ and ‘DEVELOPICKTM’ this year. Samsung Biologics will continue innovating for the successful development of new drugs and the production of high-quality biopharmaceuticals for clients.

Samsung Biologics introduced two new development technology platforms at BioProcess International 2022 between September 27-30 in Boston. The conference is the largest bioprocessing event, inviting over 1,800 experts to discuss how to accelerate promising biologics, cell and gene therapies towards commercial success.
Launching platforms S-DUALTM and DEVELOPICKTM
At this year’s event, Samsung Biologics showcased its strength in the CDO business by introducing new proprietary development technology platforms: S-DUAL™ and DEVELOPICK™.





S-DUAL™ is a high-yield bispecific antibody platform with a 99% chain-pairing success rate. Its unique asymmetrical structure reduces the risk of mispairing and ensures high binding affinity among chains to produce high titer and purity for optimized manufacturability. Bispecific antibodies are capable of targeting two different antigens at once, improving treatment potential. According to the research and consulting firm Roots Analysis, the global bispecific antibody market is expected to grow at an annual rate of 34% to reach 9.6 billion USD by 2030.
DEVELOPICK™ is a rapid developability assessment platform that systematically screens molecules at an early stage to identify candidates with the best potential for advancement to Investigational New Drug. The assessment platform enables clients to conduct a robust risk assessment for unsorted drug candidates within a month. By utilizing DEVELOPICK™, Samsung Biologics can provide early insight and selection guidance to save time and cost, and ultimately maximize efficiency.
Promoting Strengths of Differentiated CDO Services and Bolstering Networking

On September 30, Wonjun Yang, Lead Scientist for Antibody-Based Research at Samsung Biologics, shared his insights and new approaches in bispecific antibody development.
Samsung Biologics also held a total of four poster sessions to promote DEVELOPICK™ and its MSAT (Manufacturing Science and Technology) capabilities during the three-day event to showcase the company’s competency in the contract development business.



Solidifying Global Foothold as a Partner for Successful Development of New Drugs

Samsung Biologics has won over 100 projects since it first launched its CDO business in 2018. It offers end-to-end, one-stop services, from cell line development to integrated manufacturing from clinical to commercial, enabling cost and time reduction for development.
Samsung Biologics continues to strengthen its foothold in the global contract development market, by opening an R&D Center in San Francisco in 2020, launching its proprietary cell line, S-CHOice® in 2021, and the ‘S-DUALTM’ and ‘DEVELOPICKTM’ this year. Samsung Biologics will continue innovating for the successful development of new drugs and the production of high-quality biopharmaceuticals for clients.
Share article
Related Content