
Samsung Biologics is poised to complete its strategic expansion into the antibody-drug conjugate (ADC) space to address the evolving needs of clients and contribute to cancer therapy innovation. ADC therapies offer a promising alternative by precisely targeting cancer cells while minimizing the damage to healthy tissue, resulting in more effective and targeted treatments with fewer side effects in contrast to traditional chemotherapy.
With 14 ADCs already approved globally and given the huge potential of ADC therapies, Samsung Biologics is gearing up to provide ADC services by building a dedicated facility to be completed by the end of 2024 to support client pipelines, and make these transformative treatments more accessible.
Navigating the challenging ADC manufacturing landscape
ADCs deliver potent drugs directly to cancer cells via linkers attached to monoclonal antibodies, reducing the harmful side effects typically associated with chemotherapy. The toxic nature of the drugs requires strict safety protocols for the safety of scientists and staff involved, as well as the quality of the product. While ADCs represent a promising next-generation treatment, developing and manufacturing ADCs are highly complex and requires extensive expertise, advanced technologies, and improved analytical methods. Ensuring the stability and efficacy of ADCs through precise attachment of the linker and payload is key to therapeutic success, while efficient production and cost-effective scaling are also crucial for commercial viability.
As the ADC market grows, more developers are expected to rely on contract development and manufacturing organizations (CDMOs) to advance their projects. Selecting a CDMO with expertise in linker and payload technologies, along with facilities for cytotoxic drug product manufacturing, is essential to propel a program forward. With a stellar track record of success as a trusted CDMO partner, Samsung Biologics is able leverage its extensive expertise in antibody manufacturing, antibody engineering, and large-scale production, as well as cutting-edge facilities to play a pivotal role in ADC production.
Integrated expertise and cutting-edge technology
Samsung Biologics’ commitment to advancing ADC development is demonstrated through ongoing investments in its development and manufacturing capabilities. The company’s new ADC facility is designed to support the entire spectrum of ADC production, from pre-clinical to commercial manufacturing. Equipped with state-of-the-art facilities and cutting-edge technologies, Samsung Biologics is uniquely positioned to meet the intricate demands of ADC manufacturing at a single site. Manufacturing highly toxic biomedicines at a single location reduces containment risks by centralizing specialized facilities and expertise. It also minimizes transportation time and distance, streamlines the supply chain, and fosters focused research and development, leading to consistent product quality and innovation. This integrated approach ensures Samsung Biologics’ ability to offer a seamless, comprehensive ADC development program, effectively addressing key challenges.
“The complex requirements for ADCs often rely on a fragmented supply chain; therefore, it can be challenging to ensure consistent quality controls, synchronized timelines, and efficient communication across various vendors. By using a single CDMO partner, clients can simplify supply chain logistics and adopt a robust approach to eliminate bottlenecks, resulting in reliable and consistent production.” said Yoonjin Kwon, Lead Scientist in Analytical Development and a member of the company's ADC Task Force.
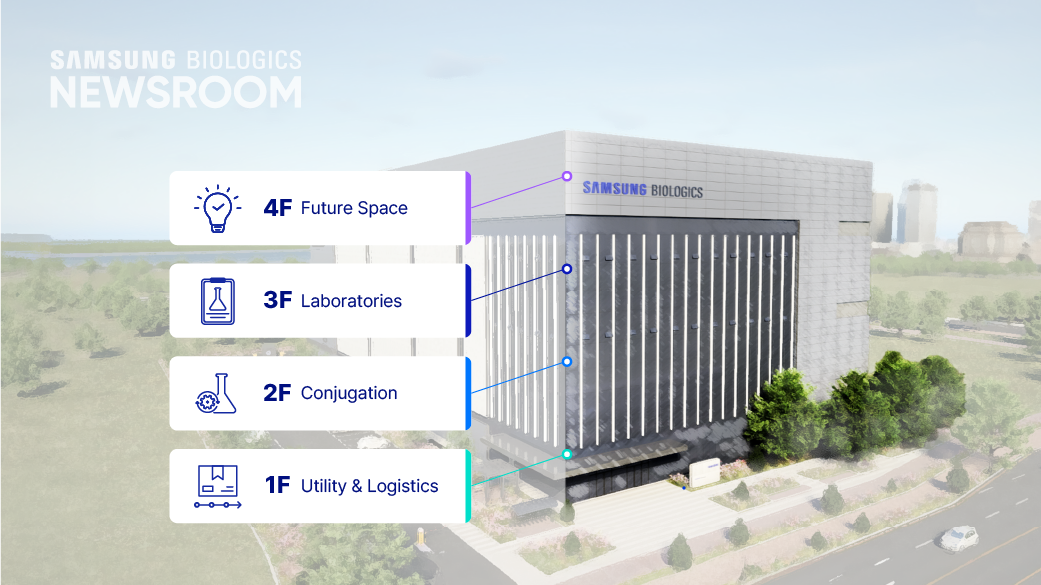
- 4F Future Space
- 3F Laboratories
- 2F Conjugation
- 1F Utility & Logistics
The facility features a flexible design accommodating both single-use and stainless steel options, capable of scaling production up to 500 liters to meet diverse client needs. It is equipped to handle Highly Potent Active Pharmaceutical Ingredients (HPAPIs), antibodies, and complex linkers with precision. Advanced isolator technology ensures stringent exposure management, safeguarding both product integrity and personnel safety, while meeting stringent occupational exposure limit (OEL) requirements for GMP production of clinical and small-scale ADC batches.
“We manage components (payload, linker and antibody) of the ADC through consistent quality and rigorous analytical development—every step of the way is validated and tested to ensure patient safety. That is always our top priority,” Kwon added.
Samsung Biologics’ commitment to innovation extends beyond its facilities and technologies. Through strategic investments through the Samsung Life Science Fund, the company is supporting Araris Biotech AG for their linker-payload toolbox and conjugation technology, as well as AimedBio for their ADC pipelines. Samsung Biologics has a partnership with LigaChem Biosciences to develop and manufacture antibody for their ADC program. These collaborations underscore Samsung Biologics’ dedication to further build on the company’s competitive edge and contribute to shaping the future of healthcare through the development of next-generation biomedicines.
In line with Samsung Biologics’ mission to create a healthier world through high-quality biomedicines, the company hopes to offer new hope for patients with its latest portfolio expansion. Samsung Biologics’ evolving capabilities and integrated approach will help enable the company to address the key challenges of ADC development and manufacturing and stay on top of latest innovations. To find out more about the ADC services Samsung Biologics will offer, please visit [LINK].

Samsung Biologics is poised to complete its strategic expansion into the antibody-drug conjugate (ADC) space to address the evolving needs of clients and contribute to cancer therapy innovation. ADC therapies offer a promising alternative by precisely targeting cancer cells while minimizing the damage to healthy tissue, resulting in more effective and targeted treatments with fewer side effects in contrast to traditional chemotherapy.
With 14 ADCs already approved globally and given the huge potential of ADC therapies, Samsung Biologics is gearing up to provide ADC services by building a dedicated facility to be completed by the end of 2024 to support client pipelines, and make these transformative treatments more accessible.
Navigating the challenging ADC manufacturing landscape
ADCs deliver potent drugs directly to cancer cells via linkers attached to monoclonal antibodies, reducing the harmful side effects typically associated with chemotherapy. The toxic nature of the drugs requires strict safety protocols for the safety of scientists and staff involved, as well as the quality of the product. While ADCs represent a promising next-generation treatment, developing and manufacturing ADCs are highly complex and requires extensive expertise, advanced technologies, and improved analytical methods. Ensuring the stability and efficacy of ADCs through precise attachment of the linker and payload is key to therapeutic success, while efficient production and cost-effective scaling are also crucial for commercial viability.
As the ADC market grows, more developers are expected to rely on contract development and manufacturing organizations (CDMOs) to advance their projects. Selecting a CDMO with expertise in linker and payload technologies, along with facilities for cytotoxic drug product manufacturing, is essential to propel a program forward. With a stellar track record of success as a trusted CDMO partner, Samsung Biologics is able leverage its extensive expertise in antibody manufacturing, antibody engineering, and large-scale production, as well as cutting-edge facilities to play a pivotal role in ADC production.
Integrated expertise and cutting-edge technology
Samsung Biologics’ commitment to advancing ADC development is demonstrated through ongoing investments in its development and manufacturing capabilities. The company’s new ADC facility is designed to support the entire spectrum of ADC production, from pre-clinical to commercial manufacturing. Equipped with state-of-the-art facilities and cutting-edge technologies, Samsung Biologics is uniquely positioned to meet the intricate demands of ADC manufacturing at a single site. Manufacturing highly toxic biomedicines at a single location reduces containment risks by centralizing specialized facilities and expertise. It also minimizes transportation time and distance, streamlines the supply chain, and fosters focused research and development, leading to consistent product quality and innovation. This integrated approach ensures Samsung Biologics’ ability to offer a seamless, comprehensive ADC development program, effectively addressing key challenges.
“The complex requirements for ADCs often rely on a fragmented supply chain; therefore, it can be challenging to ensure consistent quality controls, synchronized timelines, and efficient communication across various vendors. By using a single CDMO partner, clients can simplify supply chain logistics and adopt a robust approach to eliminate bottlenecks, resulting in reliable and consistent production.” said Yoonjin Kwon, Lead Scientist in Analytical Development and a member of the company's ADC Task Force.

- 4F Future Space
- 3F Laboratories
- 2F Conjugation
- 1F Utility & Logistics
The facility features a flexible design accommodating both single-use and stainless steel options, capable of scaling production up to 500 liters to meet diverse client needs. It is equipped to handle Highly Potent Active Pharmaceutical Ingredients (HPAPIs), antibodies, and complex linkers with precision. Advanced isolator technology ensures stringent exposure management, safeguarding both product integrity and personnel safety, while meeting stringent occupational exposure limit (OEL) requirements for GMP production of clinical and small-scale ADC batches.
“We manage components (payload, linker and antibody) of the ADC through consistent quality and rigorous analytical development—every step of the way is validated and tested to ensure patient safety. That is always our top priority,” Kwon added.
Samsung Biologics’ commitment to innovation extends beyond its facilities and technologies. Through strategic investments through the Samsung Life Science Fund, the company is supporting Araris Biotech AG for their linker-payload toolbox and conjugation technology, as well as AimedBio for their ADC pipelines. Samsung Biologics has a partnership with LigaChem Biosciences to develop and manufacture antibody for their ADC program. These collaborations underscore Samsung Biologics’ dedication to further build on the company’s competitive edge and contribute to shaping the future of healthcare through the development of next-generation biomedicines.
In line with Samsung Biologics’ mission to create a healthier world through high-quality biomedicines, the company hopes to offer new hope for patients with its latest portfolio expansion. Samsung Biologics’ evolving capabilities and integrated approach will help enable the company to address the key challenges of ADC development and manufacturing and stay on top of latest innovations. To find out more about the ADC services Samsung Biologics will offer, please visit [LINK].
Share article
Related Content