
When it comes to the challenges and trends biomanufacturers face, from market disruptions to the innovations in diverse and complex molecules, Samsung Biologics recognizes the evolving need for flexibility and quality in drug manufacturing. The company has invested in capacity and resources to ultimately give clients supply access in order to bring their product to market quickly. In this article, explore how Samsung Biologics is committed to working closely with its customers, which range from large biopharma to growing biotech companies, to deliver flexible and quality solutions that will facilitate their journey seamlessly.
Driving flexibility to manage demand
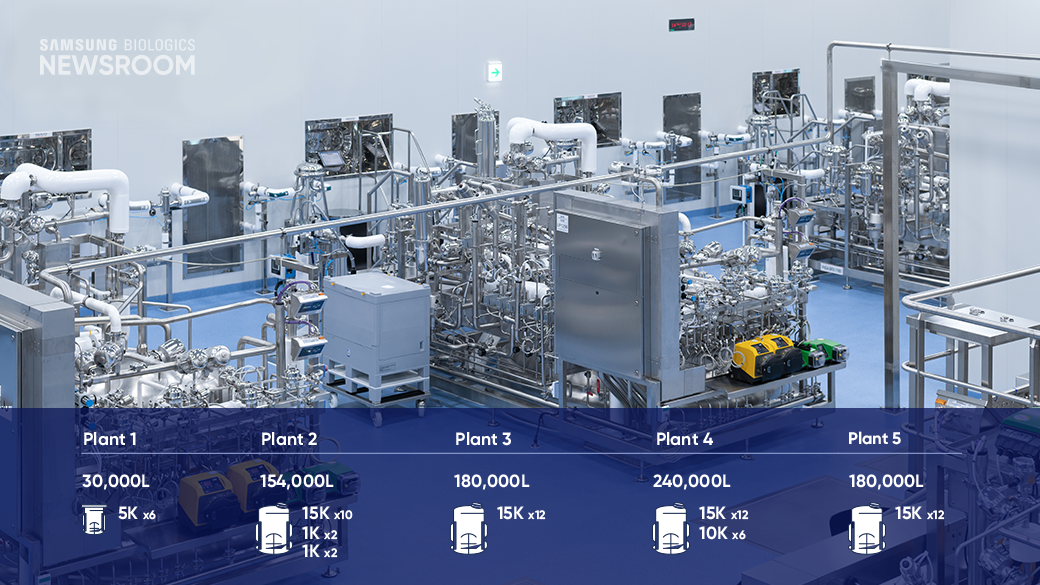
Samsung Biologics is continuously expanding its manufacturing capacity while keeping flexibility at the forefront. The company's four advanced manufacturing plants, including the newly completed Plant 4, are designed to swiftly adapt to the evolving needs of its diverse client base.
"We offer a range of bioreactor capacities from 1,000 to 15,000 liters, enabling us to provide small to large scale production. With state-of-the-art facilities and optimized timeline, we consistently produce high-quality biopharmaceuticals that meet our clients' expectations," said Soyeon Ahn, Senior Director, Plant 4 Upstream and Downstream Manufacturing.
Strong client relationships at Samsung Biologics are forged through continuous efforts to identify and address improvement points during production. Regular evaluation sessions for each project focus on enhancing equipment and processes to maximize efficiency.
"We collaborate with clients to fine-tune process like control logics of critical parameters. These improvements not only enhance yield, but also contribute to a smoother production process," Ahn added.
Ensuring quality at every phase

Quality management is a non-negotiable in biopharmaceutical manufacturing, and Samsung Biologics excels in this regard. The company has established thorough quality control measures for the entire production process, from cell culture conditions to comprehensive process monitoring and investigations.
"We're not just scaling up production; we're scaling up quality. Our commitment to quality management is unwavering, and it's reflected in every aspect of our operations," Ahn highlighted.
Samsung Biologics maintains pristine conditions at each of its manufacturing sites, regularly inspecting the equipment. "Considering that most production disruptions result from equipment issues, our well-maintained and new facilities significantly contribute to the high quality of our products," Ahn added.
Fostering an environment of continuous improvement, the MSAT, Quality, and Facility teams work tirelessly to minimize risk and maintain operational excellence.
Adopting new technologies to improve efficiency

Samsung Biologics is taking steps to leverage digitalization throughout the entire manufacturing process. This commitment to innovation extends across all facilities, resulting in continuous improvements in productivity and operational efficiency.

In Plant 4, several cutting-edge technologies were introduced to bolster efficiency and quality. A single-use alternating tangential flow (ATF) filtration system significantly reduces contamination risks when changing products, ensuring a high level of product purity and safety. The company has not only increased product yield but also cut processing times, by incorporating a continuous solids discharge centrifuge ball to separate mixture of solids.

Samsung Biologics has also introduced AxiChrom™ technology to prevent microbial contamination (bioburden) and simplify the purification column packing process.
The upcoming fifth plant, set to be completed in 2025, will further integrate automation and digital transformation throughout the entire production process to improve efficiency and consistency.

By focusing on flexibility and quality management in its biopharmaceutical manufacturing operations, Samsung Biologics remains dedicated to providing the highest standard of manufacturing excellence.

When it comes to the challenges and trends biomanufacturers face, from market disruptions to the innovations in diverse and complex molecules, Samsung Biologics recognizes the evolving need for flexibility and quality in drug manufacturing. The company has invested in capacity and resources to ultimately give clients supply access in order to bring their product to market quickly. In this article, explore how Samsung Biologics is committed to working closely with its customers, which range from large biopharma to growing biotech companies, to deliver flexible and quality solutions that will facilitate their journey seamlessly.
Driving flexibility to manage demand

Samsung Biologics is continuously expanding its manufacturing capacity while keeping flexibility at the forefront. The company's four advanced manufacturing plants, including the newly completed Plant 4, are designed to swiftly adapt to the evolving needs of its diverse client base.
"We offer a range of bioreactor capacities from 1,000 to 15,000 liters, enabling us to provide small to large scale production. With state-of-the-art facilities and optimized timeline, we consistently produce high-quality biopharmaceuticals that meet our clients' expectations," said Soyeon Ahn, Senior Director, Plant 4 Upstream and Downstream Manufacturing.
Strong client relationships at Samsung Biologics are forged through continuous efforts to identify and address improvement points during production. Regular evaluation sessions for each project focus on enhancing equipment and processes to maximize efficiency.
"We collaborate with clients to fine-tune process like control logics of critical parameters. These improvements not only enhance yield, but also contribute to a smoother production process," Ahn added.
Ensuring quality at every phase

Quality management is a non-negotiable in biopharmaceutical manufacturing, and Samsung Biologics excels in this regard. The company has established thorough quality control measures for the entire production process, from cell culture conditions to comprehensive process monitoring and investigations.
"We're not just scaling up production; we're scaling up quality. Our commitment to quality management is unwavering, and it's reflected in every aspect of our operations," Ahn highlighted.
Samsung Biologics maintains pristine conditions at each of its manufacturing sites, regularly inspecting the equipment. "Considering that most production disruptions result from equipment issues, our well-maintained and new facilities significantly contribute to the high quality of our products," Ahn added.
Fostering an environment of continuous improvement, the MSAT, Quality, and Facility teams work tirelessly to minimize risk and maintain operational excellence.
Adopting new technologies to improve efficiency

Samsung Biologics is taking steps to leverage digitalization throughout the entire manufacturing process. This commitment to innovation extends across all facilities, resulting in continuous improvements in productivity and operational efficiency.

In Plant 4, several cutting-edge technologies were introduced to bolster efficiency and quality. A single-use alternating tangential flow (ATF) filtration system significantly reduces contamination risks when changing products, ensuring a high level of product purity and safety. The company has not only increased product yield but also cut processing times, by incorporating a continuous solids discharge centrifuge ball to separate mixture of solids.

Samsung Biologics has also introduced AxiChrom™ technology to prevent microbial contamination (bioburden) and simplify the purification column packing process.
The upcoming fifth plant, set to be completed in 2025, will further integrate automation and digital transformation throughout the entire production process to improve efficiency and consistency.

By focusing on flexibility and quality management in its biopharmaceutical manufacturing operations, Samsung Biologics remains dedicated to providing the highest standard of manufacturing excellence.
Share article
Related Content