
The extraordinary speed of vaccine development in response to the COVID-19 pandemic has highlighted the unique potential of mRNA technology. The mRNA field has progressed into a promising new class of medicine to treat a wide array of unmet diseases.
Upon signing an agreement for fill-finish manufacturing of Moderna’s COVID-19 vaccine last year, Samsung Biologics has also added messenger RNA vaccine drug substance (DS) manufacturing capability to the current facility in Songdo in April this year. This has driven the company to successfully venture into offering this new technology.
In this article, we will explore what mRNA technology is and the unique capabilities Samsung Biologics can offer.
Messenger RNA carries the ‘blueprint’ instructions to produce proteins
Messenger RNA (mRNA) is a type of single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and it is made from a DNA template during the process of transcription. The role of mRNA is to carry protein information from the DNA in a cell’s nucleus to the sites of protein synthesis in the ribosomes to help make a specific protein. The ribosomes “translate” the instructions in mRNA and synthesize the protein.
*Ribosomes: A ribosome is an intercellular structure found inside the living cells which reads the messenger RNA (mRNA) sequence and translates that genetic code into a specified string of amino acids to produce proteins during a process called protein synthesis.
Promising opportunities for mRNA therapeutics
mRNA is revolutionizing the way we prevent, treat, and cure diseases, and it is opening up new ways to treat debilitating diseases such as cancer and genetic disorders.
The biggest advantages of mRNA are its flexibility to adapt to an evolving pathogen and the potential for rapid, cost-effective, and scalable manufacturing. Most mRNA-based therapeutics are currently being used against infectious diseases or to develop personalized vaccines.
Understanding mRNA COVID-19 Vaccines
Most vaccines put a weakened or inactivated germ into our bodies to stimulate an immune response. However, mRNA vaccines against COVID-19 are designed to provide our bodies with the code to produce the non-infectious virus spike protein to instruct the cell’s machinery to help trigger a natural immune response.
This response is enabled by the production of neutralizing antibodies which aims to prevent the infection. If a vaccinated person later comes into contact with the COVID-19 virus, the immune system will recognize the virus as an ‘intruder’ and enable the body to fight the virus.
The Power of One I One-stop mRNA manufacturing service provider
Frequent handling of mRNA in multiple locations increases contamination and degradation risks, but when the entire work stream from pDNA to vial is coordinated by one partner from a single location, transitions across development and production tasks run smoothly, maximizing efficiency and eliminating these potential risks.
Also, due to the unstable nature of mRNA, the need for cold-chain storage is vital when manufacturing mRNA despite the increased storage temperature by treating it with LNP (Lipid Nanoparticles) to add a protective layer.
At Samsung Biologics, we offer integrated manufacturing services from clinical to commercial, including aseptic fill-finish, labeling, packaging, and cold chain storage all at a single site to successfully manufacture mRNA therapeutics.
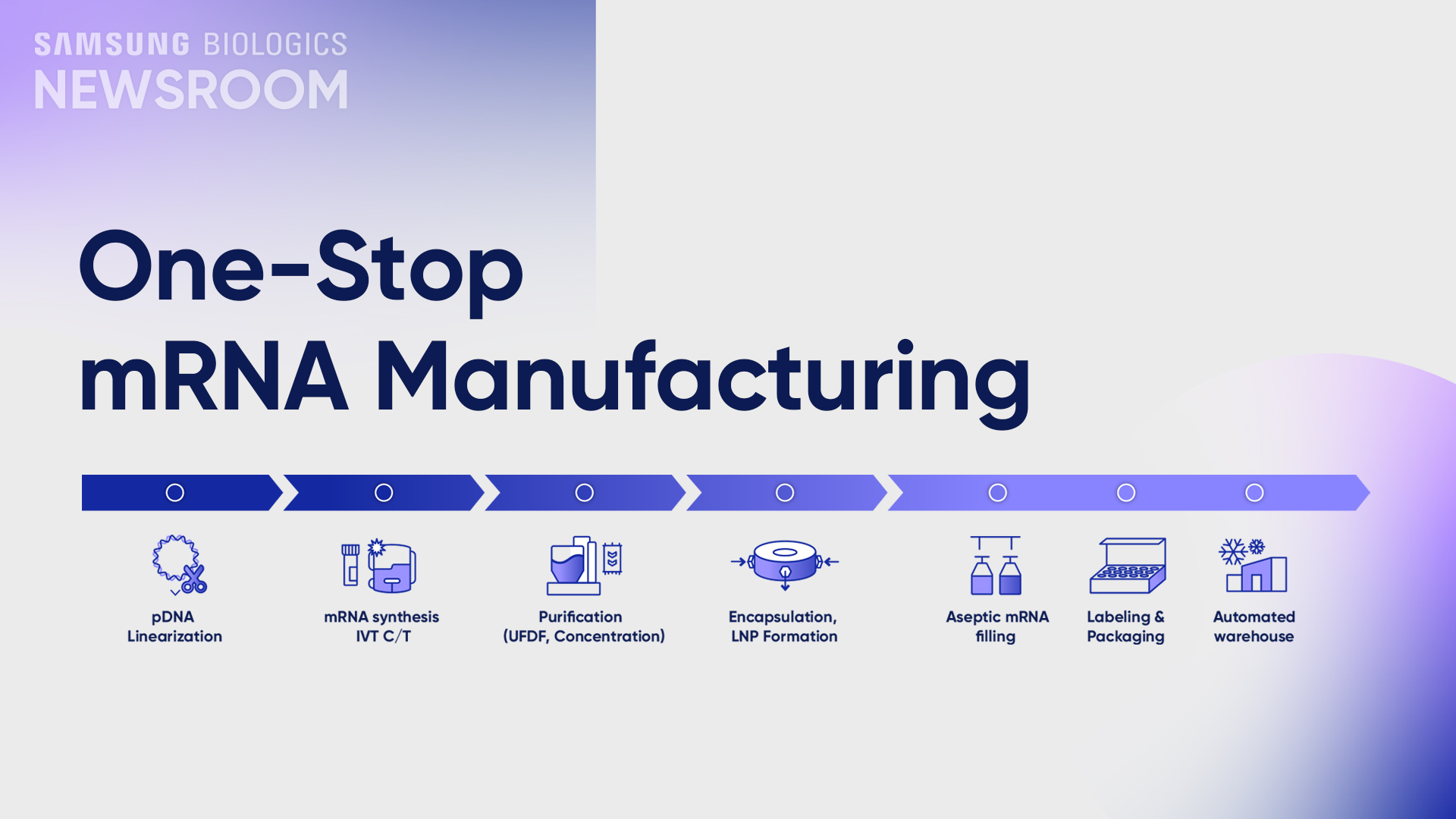
As a one-stop mRNA manufacturing partner, we are fully equipped with an mRNA dedicated MSAT lab where we offer a one-stop solution to facilitate our client’s molecules to commercial through tech transfer, process, development, and process characterization.

Forging ahead toward the next breakthrough
As part of the company’s sustainable growth plan, we are continuing with our advancements in business portfolio, venturing into offering multi-modal products including cell & gene therapies and next-gen vaccines utilizing mRNA, pDNA, and viral vectors, all at a single site.
Continuing from our partnership signing with Moderna and GreenLight Biosciences to manufacture COVID-19 vaccines, our teams of experts will forge ahead together with our efforts to proactively grow into a manufacturing hub for vaccines to help save lives of people worldwide.
Together, let’s forge ahead towards the next breakthrough.
Driven. For Life.
Related contents
Samsung BIO Insight The Benefit of Speed, Scale & Quality | Explore Samsung Biologics' CMO Strengths
SamsungBIO Insight The Journey to Your Success | Explore Samsung Biologics' CDO Strengths
SamsungBIO Insight A Strategic Roadmap for Sustainable Growth - Samsung Biologics at J.P. Morgan Healthcare Conference 2022

The extraordinary speed of vaccine development inresponse to the COVID-19 pandemic has highlighted the unique potential of mRNA technology. The mRNA field has progressed into a promising new class of medicine to treat a wide array of unmet diseases.
Upon signing an agreement for fill-finish manufacturing of Moderna’s COVID-19 vaccine last year, Samsung Biologics has also added messenger RNA vaccine drug substance (DS) manufacturing capability to the current facility in Songdo in April this year. This has driven the company to successfully venture into offering this new technology.
In this article, we will explore what mRNA technology is and the unique capabilities Samsung Biologics can offer.
Messenger RNA carries the ‘blueprint’ instructions to produce proteins
Messenger RNA (mRNA) is a type ofsingle-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and it is made from a DNA template during the process of transcription. The role of mRNA is to carry protein information from the DNA in a cell’s nucleus to the sites of protein synthesis in the ribosomes to help make a specific protein. The ribosomes “translate” the instructions in mRNA and synthesize the protein.
* Ribosomes: A ribosome is an intercellular structure found inside the living cells which reads the messenger RNA (mRNA) sequence and translates that genetic code into a specified string of amino acids to produce proteins during a process called protein synthesis.
Promising opportunities form RNA therapeutics
mRNA is revolutionizing the way we prevent, treat, and cure diseases, and it is opening up new ways to treat debilitating diseases such as cancer andgenetic disorders.
The biggest advantages of mRNA are its flexibility to adapt to an evolving pathogen and the potential for rapid, cost-effective, and scalable manufacturing. Most mRNA-based therapeutics are currently being used against infectious diseases or to develop personalized vaccines.
Understanding mRNA COVID-19 Vaccines
Most vaccines put a weakened or inactivated germ into our bodies to stimulate an immune response. However, mRNA vaccines against COVID-19 are designedto provide our bodies with the code to produce the non-infectious virus spike protein to instruct the cell’s machinery to help trigger a natural immune response.
This response is enabled by the production of neutralizing antibodies which aims to prevent the infection. If a vaccinated person later comes into contact with the COVID-19 virus, the immune system will recognize the virus as an ‘intruder’ and enable the body to fight the virus.

The Power of One I One-stop mRNA manufacturing service provider
Frequent handling of mRNA in multiple locations increases contamination and degradation risks, but when the entire work stream from pDNA to vial is coordinated by one partner from a single location, transitions across development and production tasks run smoothly, maximizing efficiency and eliminating these potential risks.
Also, due to the unstable nature of mRNA, the need for cold-chain storage is vital when manufacturing mRNA despitethe increased storage temperature by treating it with LNP (Lipid Nanoparticles) to add a protective layer.
At Samsung Biologics, we offer integrated manufacturing services from clinical to commercial, including aseptic fill-finish, labeling, packaging, and cold chain storage all at a single site to successfully manufacture mRNA therapeutics.

As a one-stop mRNA manufacturing partner, we are fully equipped with an mRNA dedicated MSAT lab where we offer a one-stop solution to facilitate our client’s molecules to commercial through tech transfer, process, development, and process characterization.

Forging ahead toward the next breakthrough
As part of the company’s sustainable growth plan, we are continuing with our advancements in business portfolio, venturing into offering multi-modal products including cell & gene therapies and next-gen vaccines utilizing mRNA, pDNA, andviral vectors, all at a single site.
Continuing from our partnership signing with Moderna and GreenLight Biosciences to manufacture COVID-19 vaccines, our teams of experts will forge ahead together with our efforts to proactively grow into a manufacturing hub for vaccines to help save lives of people worldwide.
Together, let’s forge ahead towards the next breakthrough.
Driven. For Life.
Related contents
SamsungBIO Insight The Benefit of Speed, Scale & Quality | Explore Samsung Biologics' CMO Strengths
SamsungBIO Insight The Journey to Your Success | Explore Samsung Biologics' CDO Strengths
SamsungBIO Insight A Strategic Roadmap for Sustainable Growth - Samsung Biologics at J.P. Morgan Healthcare Conference 2022
- CDO
- CGMP
- ADC
- Bio Campus
- IR
- CMO
Share article