CEO MESSAGE Driven. For Life.
Announcement
Dear valued investors and shareholders, This is John Rim from Samsung Biologics. To begin, I would like to extend my heartfelt appreciation for your continued interest and steadfast support of our business.
Key Highlights
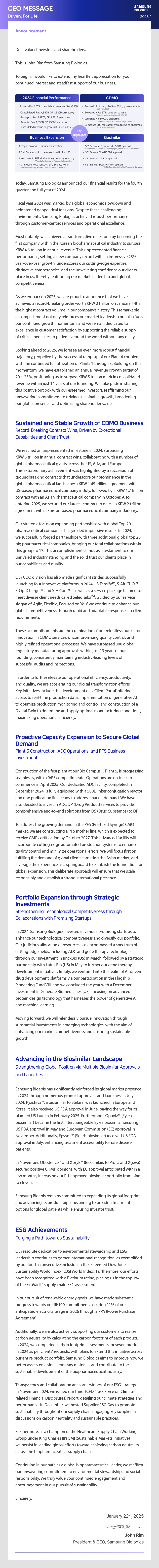
- 2024 Financial Performance
- • Posted KRW 4.5T in consolidated revenue (YoY +23%) -Consolidated: Rev. 4,547B, OP. 1,320B (OPM: 29.0%) - Biologics: Rev. 3,497B, OP. 1,321B (OPM: 37.8%) - Bioepis: Rev. 1,538B, OP. 435B (OPM: 28.3%)
- • Consolidated revenue to grow +20-25% in 2025
- CDMO
- Secured 17 of the global top 20 big pharma clients *14 clients in 2023
- • Exceeded KRW 5T in contract volume *Signed 3 major contracts worth KRW 1T+
- • Launched 4 new CDO platforms *S-TensifyTM, S-AfuCHOTM, S-OptiChargeTM, S-HiconTM
- • Surpassed 300 regulatory manufacturing approvals *340 approvals in total
- Business Expansion
- • Completion of ADC facility construction
- • P5 at Biocampus II to be operational in Apr. '25
- • Investment in PFS Mother-line (CGMP ready by Oct. 27) *Foundational set-up for future global expansion
- • Continued investments via Life Science Fund *Flagship Pioneening, BrickBio, Latus Bio, Generate Biomedicines
- Biosimilar
- • SB17 (bStelara): EU launch & US FDA approval * Launched in EU, including Germany, Spain, France and the Netherlands
- • SB15 (bEylea):EC & US FDA approval
- • SB12 (bSoliris) :US FDA approval
- • SB16 (bProlia):Positive CHMP opinion *EMA's Committee for Medicinal Products for Human Use
Today, Samsung Biologics announced our financial results for the fourth quarter and full year of 2024.
Fiscal year 2024 was marked by a global economic slowdown and heightened geopolitical tensions. Despite these challenging environments, Samsung Biologics achieved robust performance through customer-centric services and operational excellence.
Most notably, we achieved a transformative milestone by becoming the first company within the Korean biopharmaceutical industry to surpass KRW 4.5 trillion in annual revenue. This unprecedented financial performance, setting a new company record with an impressive 23% year-over-year growth, underscores our cutting-edge expertise, distinctive competencies, and the unwavering confidence our clients place in us, thereby reaffirming our market leadership and global competitiveness.
As we embark on 2025, we are proud to announce that we have achieved a record-breaking order worth KRW 2 trillion on January 14th, the highest contract volume in our company's history. This remarkable accomplishment not only reinforces our market leadership but also fuels our continued growth momentum, and we remain dedicated to excellence in customer satisfaction by supporting the reliable supply of critical medicines to patients around the world without any delay.
Looking ahead to 2025, we foresee an even more robust financial trajectory, propelled by the successful ramp-up of our Plant 4 coupled with the continued full utilization of Plants 1 through 3. Building on this momentum, we have established an annual revenue growth target of 20~25%, positioning us to surpass KRW 5 trillion mark in consolidated revenue within just 14 years of our founding. We take pride in sharing this positive outlook with our esteemed investors, reaffirming our unwavering commitment to driving sustainable growth, broadening our global presence, and optimizing shareholder value.
Sustained and Stable Growth of CDMO Business Record-Breaking Contract Wins, Driven by Exceptional Capabilities and Client Trust
We reached an unprecedented milestone in 2024, surpassing KRW 5 trillion in annual contract wins, collaborating with a number of global pharmaceutical giants across the US, Asia, and Europe. This extraordinary achievement was highlighted by a succession of groundbreaking contracts that underscore our prominence in the global pharmaceutical landscape: a KRW 1.45 trillion agreement with a US-based pharmaceutical company in July, followed by a KRW 1.7 trillion contract with an Asian pharmaceutical company in October. Also, entering 2025, we secured our largest contract to date - a KRW 2 trillion agreement with a Europe-based pharmaceutical company in January.
Our strategic focus on expanding partnerships with global Top 20 pharmaceutical companies has yielded impressive results. In 2024, we successfully forged partnerships with three additional global top 20 big pharmaceutical companies, bringing our total collaborations within this group to 17. This accomplishment stands as a testament to our unrivaled industry standing and the solid trust our clients place in our capabilities and quality.
Our CDO division has also made significant strides, successfully launching four innovative platforms in 2024 - S-TensifyTM, S-AfuCHOTM, S-OptiChargeTM, and S-HiConTM - as well as a service package tailored to meet diverse client needs called SelecTailorTM. Guided by our service slogan of 'Agile, Flexible, Focused on You, we continue to enhance our global competitiveness through rapid and adaptable responses to client requirements.
These accomplishments are the culmination of our relentless pursuit of innovation in CDMO services, uncompromising quality control, and highly refined operational processes. We have surpassed 300 global regulatory manufacturing approvals within just 13 years of our founding, consistently maintaining industry-leading levels of successful audits and inspections.
In order to further elevate our operational efficiency, productivity, and quality, we are accelerating our digital transformation efforts. Key initiatives include the development of a 'Client Portal' offering access to real-time production data; implementation of generative Al to optimize production monitoring and control; and construction of a Digital Twin to determine and apply optimal manufacturing conditions, maximizing operational efficiency.
Proactive Capacity Expansion to Secure Global Demand
Plant 5 Construction, ADC Operations, and PFS Business Investment
Construction of the first plant at our Bio Campus II, Plant 5, is progressing
seamlessly, with a 94% completion rate. Operations are on track to commence in April 2025. Our dedicated ADC facility, completed in December 2024, is fully equipped with a 500L linker conjugation reactor and one purification line, ready to address market demand. We have also decided to invest in ADC DP (Drug Product) services to provide comprehensive end-to-end solutions from DS (Drug Substance) to DP.
To address the growing demand in the PFS (Pre-filled Syringe) CMO market, we are constructing a PFS mother line, which is expected to receive GMP certification by October 2027. This advanced facility will incorporate cutting-edge automated production systems to enhance quality control and minimize operational errors. We will focus first on fulfilling the demand of global clients targeting the Asian market, and leverage the experience as a springboard to establish the foundation for global expansion. This deliberate approach will ensure that we scale responsibly and establish a strong international presence.
Portfolio Expansion through Strategic
Investments
Strengthening Technological Competitiveness through Collaborations with Promising Startups
In 2024, Samsung Biologics invested in various promising startups to enhance our technological competitiveness and diversify our portfolio. Our judicious allocation of resources has encompassed a spectrum of cutting-edge fields, including ADC and gene therapy technologies through our investment in BrickBio (US) in March, followed by a strategic partnership with Latus Bio (US) in May to further our gene therapy development initiatives. In July, we ventured into the realm of Al-driven drug development platforms via our participation in the Flagship Pioneering Fund VIII, and we concluded the year with a December investment in Generate Biomedicines (US), focusing on advanced protein design technology that harnesses the power of generative Al and machine learning.
Moving forward, we will relentlessly pursue innovation through substantial investments in emerging technologies, with the aim of enhancing our market competitiveness and ensuring sustainable
growth.
Advancing in the Biosimilar Landscape Strengthening Global Position via Multiple Biosimilar Approvals
and Launches
Samsung Bioepis has significantly reinforced its global market presence
in 2024 through numerous product approvals and launches. In July
2024, PyzchivaTM, a biosimilar to Stelara, was launched in Europe and
Korea. It also received US FDA approval in June, paving the way for its planned US launch in February 2025. Furthermore, OpuvizTM (Eylea biosimilar) became the first interchangeable Eylea biosimilar, securing US FDA approval in May and European Commission (EC) approval in November. Additionally, EpysqliTM (Soliris biosimilar) received US FDA approval in July, enhancing treatment accessibility for rare disease
patients.
In November, ObodenceTM and XbrykTM (Biosimilars to Prolia and Xgeva) secured positive CHMP opinions, with EC approval anticipated within a few months, increasing our EU-approved biosimilar portfolio from nine
to eleven.
Samsung Bioepis remains committed to expanding its global footprint and advancing its product pipeline, aiming to broaden treatment options for global patients while ensuring investor trust.
ESG Achievements
Forging a Path towards Sustainability
Our resolute dedication to environmental stewardship and ESG
leadership continues to garner international recognition, as exemplified by our fourth consecutive inclusion in the esteemed Dow Jones Sustainability World Index (DJSI World Index). Furthermore, our efforts have been recognized with a Platinum rating, placing us in the top 1% of the EcoVadis' supply chain ESG assessment.
In our pursuit of renewable energy goals, we have made substantial progress towards our RE100 commitment, securing 11% of our anticipated electricity usage in 2026 through a PPA (Power Purchase
Agreement).
Additionally, we are also actively supporting our customers to realize carbon neutrality by calculating the carbon footprint of each product. In 2024, we completed carbon footprint assessments for seven products in 2024 as per clients' requests, with plans to extend this initiative across our entire product portfolio. Samsung Biologics aims to improve how we better assess emissions from raw materials and contribute to the sustainable development of the biopharmaceutical industry.
Transparency and collaboration are cornerstones of our ESG strategy.
In November 2024, we issued our third TCFD (Task Force on Climate- related Financial Disclosures) report, detailing our climate strategies and performance. In December, we hosted Supplier ESG Day to promote sustainability throughout our supply chain, engaging key suppliers in discussions on carbon neutrality and sustainable practices.
Furthermore, as a champion of the Healthcare Supply Chain Working Group under King Charles III's SMI (Sustainable Markets Initiative) we persist in leading global efforts toward achieving carbon neutrality across the biopharmaceutical supply chain.
Continuing in our path as a global biopharmaceutical leader, we reaffirm our unwavering commitment to environmental stewardship and social responsibility. We truly value your continued engagement and encouragement in our pursuit of sustainability.
Sincerely,
January 22nd, 2025
John Rim President & CEO, Samsung Biologics
CEO MESSAGE Driven. For Life.
Announcement
Dear valued investors and shareholders, This is John Rim from Samsung Biologics. To begin, I would like to extend my heartfelt appreciation for your continued interest and steadfast support of our business.
Key Highlights
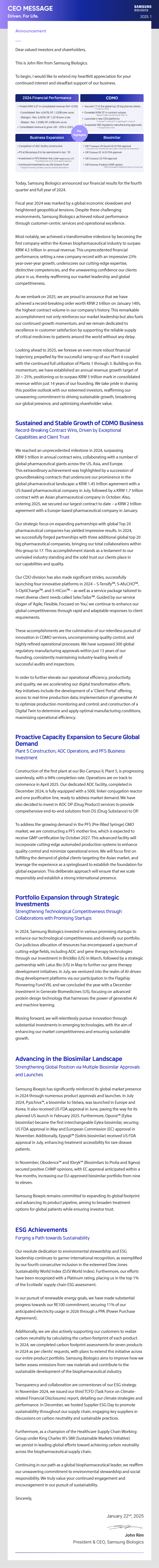
- 2024 Financial Performance
- • Posted KRW 4.5T in consolidated revenue (YoY +23%) -Consolidated: Rev. 4,547B, OP. 1,320B (OPM: 29.0%) - Biologics: Rev. 3,497B, OP. 1,321B (OPM: 37.8%) - Bioepis: Rev. 1,538B, OP. 435B (OPM: 28.3%)
- • Consolidated revenue to grow +20-25% in 2025
- CDMO
- Secured 17 of the global top 20 big pharma clients *14 clients in 2023
- • Exceeded KRW 5T in contract volume *Signed 3 major contracts worth KRW 1T+
- • Launched 4 new CDO platforms *S-TensifyTM, S-AfuCHOTM, S-OptiChargeTM, S-HiconTM
- • Surpassed 300 regulatory manufacturing approvals *340 approvals in total
- Business Expansion
- • Completion of ADC facility construction
- • P5 at Biocampus II to be operational in Apr. '25
- • Investment in PFS Mother-line (CGMP ready by Oct. 27) *Foundational set-up for future global expansion
- • Continued investments via Life Science Fund *Flagship Pioneening, BrickBio, Latus Bio, Generate Biomedicines
- Biosimilar
- • SB17 (bStelara): EU launch & US FDA approval * Launched in EU, including Germany, Spain, France and the Netherlands
- • SB15 (bEylea):EC & US FDA approval
- • SB12 (bSoliris) :US FDA approval
- • SB16 (bProlia):Positive CHMP opinion *EMA's Committee for Medicinal Products for Human Use
Today, Samsung Biologics announced our financial results for the fourth quarter and full year of 2024.
Fiscal year 2024 was marked by a global economic slowdown and heightened geopolitical tensions. Despite these challenging environments, Samsung Biologics achieved robust performance through customer-centric services and operational excellence.
Most notably, we achieved a transformative milestone by becoming the first company within the Korean biopharmaceutical industry to surpass KRW 4.5 trillion in annual revenue. This unprecedented financial performance, setting a new company record with an impressive 23% year-over-year growth, underscores our cutting-edge expertise, distinctive competencies, and the unwavering confidence our clients place in us, thereby reaffirming our market leadership and global competitiveness.
As we embark on 2025, we are proud to announce that we have achieved a record-breaking order worth KRW 2 trillion on January 14th, the highest contract volume in our company's history. This remarkable accomplishment not only reinforces our market leadership but also fuels our continued growth momentum, and we remain dedicated to excellence in customer satisfaction by supporting the reliable supply of critical medicines to patients around the world without any delay.
Looking ahead to 2025, we foresee an even more robust financial trajectory, propelled by the successful ramp-up of our Plant 4 coupled with the continued full utilization of Plants 1 through 3. Building on this momentum, we have established an annual revenue growth target of 20~25%, positioning us to surpass KRW 5 trillion mark in consolidated revenue within just 14 years of our founding. We take pride in sharing this positive outlook with our esteemed investors, reaffirming our unwavering commitment to driving sustainable growth, broadening our global presence, and optimizing shareholder value.
Sustained and Stable Growth of CDMO Business Record-Breaking Contract Wins, Driven by Exceptional Capabilities and Client Trust
We reached an unprecedented milestone in 2024, surpassing KRW 5 trillion in annual contract wins, collaborating with a number of global pharmaceutical giants across the US, Asia, and Europe. This extraordinary achievement was highlighted by a succession of groundbreaking contracts that underscore our prominence in the global pharmaceutical landscape: a KRW 1.45 trillion agreement with a US-based pharmaceutical company in July, followed by a KRW 1.7 trillion contract with an Asian pharmaceutical company in October. Also, entering 2025, we secured our largest contract to date - a KRW 2 trillion agreement with a Europe-based pharmaceutical company in January.
Our strategic focus on expanding partnerships with global Top 20 pharmaceutical companies has yielded impressive results. In 2024, we successfully forged partnerships with three additional global top 20 big pharmaceutical companies, bringing our total collaborations within this group to 17. This accomplishment stands as a testament to our unrivaled industry standing and the solid trust our clients place in our capabilities and quality.
Our CDO division has also made significant strides, successfully launching four innovative platforms in 2024 - S-TensifyTM, S-AfuCHOTM, S-OptiChargeTM, and S-HiConTM - as well as a service package tailored to meet diverse client needs called SelecTailorTM. Guided by our service slogan of 'Agile, Flexible, Focused on You, we continue to enhance our global competitiveness through rapid and adaptable responses to client requirements.
These accomplishments are the culmination of our relentless pursuit of innovation in CDMO services, uncompromising quality control, and highly refined operational processes. We have surpassed 300 global regulatory manufacturing approvals within just 13 years of our founding, consistently maintaining industry-leading levels of successful audits and inspections.
In order to further elevate our operational efficiency, productivity, and quality, we are accelerating our digital transformation efforts. Key initiatives include the development of a 'Client Portal' offering access to real-time production data; implementation of generative Al to optimize production monitoring and control; and construction of a Digital Twin to determine and apply optimal manufacturing conditions, maximizing operational efficiency.
Proactive Capacity Expansion to Secure Global Demand
Plant 5 Construction, ADC Operations, and PFS Business Investment
Construction of the first plant at our Bio Campus II, Plant 5, is progressing
seamlessly, with a 94% completion rate. Operations are on track to commence in April 2025. Our dedicated ADC facility, completed in December 2024, is fully equipped with a 500L linker conjugation reactor and one purification line, ready to address market demand. We have also decided to invest in ADC DP (Drug Product) services to provide comprehensive end-to-end solutions from DS (Drug Substance) to DP.
To address the growing demand in the PFS (Pre-filled Syringe) CMO market, we are constructing a PFS mother line, which is expected to receive GMP certification by October 2027. This advanced facility will incorporate cutting-edge automated production systems to enhance quality control and minimize operational errors. We will focus first on fulfilling the demand of global clients targeting the Asian market, and leverage the experience as a springboard to establish the foundation for global expansion. This deliberate approach will ensure that we scale responsibly and establish a strong international presence.
Portfolio Expansion through Strategic
Investments
Strengthening Technological Competitiveness through Collaborations with Promising Startups
In 2024, Samsung Biologics invested in various promising startups to enhance our technological competitiveness and diversify our portfolio. Our judicious allocation of resources has encompassed a spectrum of cutting-edge fields, including ADC and gene therapy technologies through our investment in BrickBio (US) in March, followed by a strategic partnership with Latus Bio (US) in May to further our gene therapy development initiatives. In July, we ventured into the realm of Al-driven drug development platforms via our participation in the Flagship Pioneering Fund VIII, and we concluded the year with a December investment in Generate Biomedicines (US), focusing on advanced protein design technology that harnesses the power of generative Al and machine learning.
Moving forward, we will relentlessly pursue innovation through substantial investments in emerging technologies, with the aim of enhancing our market competitiveness and ensuring sustainable
growth.
Advancing in the Biosimilar Landscape Strengthening Global Position via Multiple Biosimilar Approvals
and Launches
Samsung Bioepis has significantly reinforced its global market presence
in 2024 through numerous product approvals and launches. In July
2024, PyzchivaTM, a biosimilar to Stelara, was launched in Europe and
Korea. It also received US FDA approval in June, paving the way for its planned US launch in February 2025. Furthermore, OpuvizTM (Eylea biosimilar) became the first interchangeable Eylea biosimilar, securing US FDA approval in May and European Commission (EC) approval in November. Additionally, EpysqliTM (Soliris biosimilar) received US FDA approval in July, enhancing treatment accessibility for rare disease
patients.
In November, ObodenceTM and XbrykTM (Biosimilars to Prolia and Xgeva) secured positive CHMP opinions, with EC approval anticipated within a few months, increasing our EU-approved biosimilar portfolio from nine
to eleven.
Samsung Bioepis remains committed to expanding its global footprint and advancing its product pipeline, aiming to broaden treatment options for global patients while ensuring investor trust.
ESG Achievements
Forging a Path towards Sustainability
Our resolute dedication to environmental stewardship and ESG
leadership continues to garner international recognition, as exemplified by our fourth consecutive inclusion in the esteemed Dow Jones Sustainability World Index (DJSI World Index). Furthermore, our efforts have been recognized with a Platinum rating, placing us in the top 1% of the EcoVadis' supply chain ESG assessment.
In our pursuit of renewable energy goals, we have made substantial progress towards our RE100 commitment, securing 11% of our anticipated electricity usage in 2026 through a PPA (Power Purchase
Agreement).
Additionally, we are also actively supporting our customers to realize carbon neutrality by calculating the carbon footprint of each product. In 2024, we completed carbon footprint assessments for seven products in 2024 as per clients' requests, with plans to extend this initiative across our entire product portfolio. Samsung Biologics aims to improve how we better assess emissions from raw materials and contribute to the sustainable development of the biopharmaceutical industry.
Transparency and collaboration are cornerstones of our ESG strategy.
In November 2024, we issued our third TCFD (Task Force on Climate- related Financial Disclosures) report, detailing our climate strategies and performance. In December, we hosted Supplier ESG Day to promote sustainability throughout our supply chain, engaging key suppliers in discussions on carbon neutrality and sustainable practices.
Furthermore, as a champion of the Healthcare Supply Chain Working Group under King Charles III's SMI (Sustainable Markets Initiative) we persist in leading global efforts toward achieving carbon neutrality across the biopharmaceutical supply chain.
Continuing in our path as a global biopharmaceutical leader, we reaffirm our unwavering commitment to environmental stewardship and social responsibility. We truly value your continued engagement and encouragement in our pursuit of sustainability.
Sincerely,
January 22nd, 2025
John Rim President & CEO, Samsung Biologics
- CDO
- CGMP
- ADC
- Bio Campus
- IR
- CMO