CEO IR NEWSLETTER Driven. For Life.
SAMSUNG BIOLOGICS 2024.04
Dear valued investors and shareholders, This is John Rim from Samsung Biologics. First, I would like to express my gratitude for your continued interest and support this year.
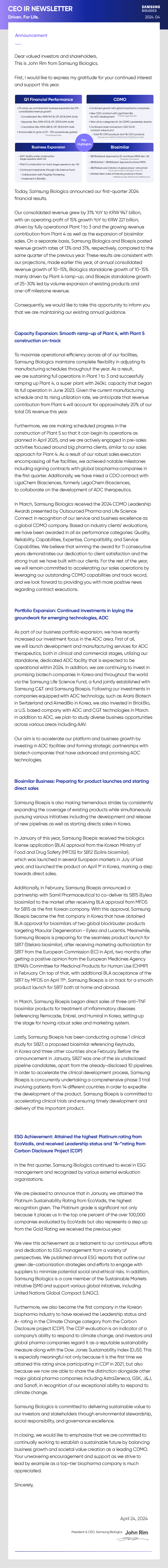
Key Highlights
- Q1 Financial Performance
- . P4 ramp-up and biosimilar business expansion led 31% consolidated revenue growth -Consolidated: Rev. KRW 947 B, OP. 221 B (OPM: 23.4%) -Separate: Rev. KRW 670 B, OP. 233 B (OPM: 34.8%) -Cumulative: Rev. KRW 280 B, OP. 38 B (OPM: 13.6%) . Annual sales to grow at 10 - 15% as previously guided * Consolidated basis
- CDMO
- . Continued growth with global biopharma companies . New CDO contract with LigaChem Bio *Former LegoChem Bio . Won all six categories for '24 CDMO Leadership Awards . Continued order momentum: USD 12.5 B contract value (cum.) - Total 90 CMO products and 116 CDO products * Secured one additional Global Top 20 Big Pharma client
- Business Expansion
- . ADC facility under construction (target operation within '24) . Plant 5 construction on-track (target operation in Apr. '25) . Continued investments through Life Science Fund: - Collaboration with Flagship Pioneering - Investment in BrickBio
- Biosimilar
- . SB17(bStelara): Approved by EC and Korea MFDS (Apr. '24) *European Commission . SB12(bSoliris) / SB15(bEylea): Approved by Korea MFDS . SB27(bKeytruda): Initiation of global phase 1 clinical trial * Concurrent phase 3 trial began in Apr. '24 . Initiated direct sales of three key products in Korea *SB2[bRemicade), SB4(bEnbrel), SB5(bHumira)
Today, Samsung Biologics announced our first-quarter 2024 financial results. Our consolidated revenue grew by 31% YoY to KRW 947 billion, with an operating profit of 15% growth YoY to KRW 221 billion, driven by fully operational Plant 1 to 3 and the growing revenue contribution from Plant 4 as well as the expansion of biosimilar sales. On a separate basis, Samsung Biologics and Bioepis posted revenue growth rates of 13% and 31%, respectively, compared to the same quarter of the previous year. These results are consistent with our projections, made earlier this year, of annual consolidated revenue growth of 10-15%, Biologics standalone growth of 10-15% mainly driven by Plant 4 ramp-up, and Bioepis standalone growth of 25-30% led by volume expansion of existing products and one-off milestone revenue. Consequently, we would like to take this opportunity to inform you that we are maintaining our existing annual guidance.
Capacity Expansion: Smooth ramp-up of Plant 4, with Plant 5 construction on-track
To maximize operational efficiency across all of our facilities, Samsung Biologics maintains complete flexibility in adjusting its manufacturing schedules throughout the year. As a result, we are sustaining full operations in Plant 1 to 3 and successfully ramping up Plant 4, a super plant with 240kL capacity that began its full operation in June 2023. Given the current manufacturing schedule and its rising utilization rate, we anticipate that revenue contribution from Plant 4 will account for approximately 20% of our total DS revenue this year. Furthermore, we are making scheduled progress in the construction of Plant 5 so that it can begin its operations as planned in April 2025, and we are actively engaged in pre-sales activities focused around big pharma clients, similar to our sales approach for Plant 4. As a result of our robust sales execution encompassing all five facilities, we achieved notable milestones including signing contracts with global biopharma companies in the first quarter. Additionally, we have inked a CDO contract with LigaChem Biosciences, formerly LegoChem Biosciences, to collaborate on the development of ADC therapeutics. In March, Samsung Biologics received the 2024 CDMO Leadership Awards presented by Outsourced Pharma and Life Science Connect in recognition of our service and business excellence as a global CDMO company. Based on industry clients' evaluations, we have been awarded in all six performance categories: Quality, Reliability, Capabilities, Expertise, Compatibility, and Service Capabilities. We believe that winning the award for 11 consecutive years demonstrates our dedication to client satisfaction and the strong trust we have built with our clients. For the rest of the year, we will remain committed to accelerating our sales operations by leveraging our outstanding CDMO capabilities and track record, and we look forward to providing you with more positive news regarding contract executions.
Portfolio Expansion: Continued investments in laying the groundwork for emerging technologies, ADC
As part of our business portfolio expansion, we have recently increased our investment focus in the ADC area. First of all, we will launch development and manufacturing services for ADC therapeutics, both in clinical and commercial stages, utilizing our standalone, dedicated ADC facility that is expected to be operational within 2024. In addition, we are continuing to invest in promising biotech companies in Korea and throughout the world via the Samsung Life Science Fund, a fund jointly established with Samsung C&T and Samsung Bioepis. Following our investments in companies equipped with ADC technology, such as Araris Biotech in Switzerland and AimedBio in Korea, we also invested in BrickBio, a U.S. based company with ADC and CGT technologies in March. In addition to ADC, we plan to study diverse business opportunities across various areas including AAV. Our aim is to accelerate our platform and business growth by investing in ADC facilities and forming strategic partnerships with biotech companies that have advanced and promising ADC technologies.
Biosimilar Business: Preparing for product launches and starting direct sales
Samsung Bioepis is also making tremendous strides by consistently expanding the coverage of existing products while simultaneously pursuing various initiatives including the development and release of new pipelines as well as starting directs sales in Korea. In January of this year, Samsung Bioepis received the biologics license application (BLA) approval from the Korean Ministry of Food and Drug Safety (MFDS) for SB12 (Soliris biosimilar), which was launched in several European markets in July of last year, and launched the product on April 1st in Korea, marking a step towards direct sales. Additionally, in February, Samsung Bioepis announced a partnership with Samil Pharmaceutical to co-deliver its SB15 (Eylea biosimilar) to the market after receiving BLA approval from MFDS for SB15 as the first Korean company. With this approval, Samsung Bioepis became the first company in Korea that have obtained BLA approval for biosimilars of two global blockbuster products targeting Macular Degeneration - Eylea and Lucentis. Meanwhile, Samsung Bioepis is preparing for the seamless product launch for SB17 (Stelara biosimilar), after receiving marketing authorization for SB17 from the European Commission (EC) in April, two months after getting a positive opinion from the European Medicines Agency (EMA)'s Committee for Medicinal Products for Human Use (CHMP) in February. On top of that, with additional BLA acceptance of the SB17 by MFDS on April 11th, Samsung Bioepis is on track for a smooth product launch for SB17 both at home and abroad. In March, Samsung Bioepis began direct sales of three anti-TNF biosimilar products for treatment of inflammatory diseases (referencing Remicade, Enbrel, and Humira) in Korea, setting up the stage for having robust sales and marketing system. Lastly, Samsung Bioepis has been conducting a phase 1 clinical study for SB27, a proposed biosimilar referencing Keytruda, in Korea and three other countries since February. Before the announcement in January, SB27 was one of the six undisclosed pipeline candidates, apart from the already-disclosed 10 pipelines. In order to accelerate the clinical development process, Samsung Bioepis is concurrently undertaking a comprehensive phase 3 trial involving patients from 14 different countries in order to expedite the development of the product. Samsung Bioepis is committed to accelerating clinical trials and ensuring timely development and delivery of this important product.
ESG Achievement: Attained the highest Platinum rating from EcoVadis, and received Leadership status and "A-"rating from Carbon Disclosure Project (CDP)
In the first quarter, Samsung Biologics continued to excel in ESG management and recognized by various external evaluation organizations. We are pleased to announce that in January, we attained the Platinum Sustainability Rating from EcoVadis, the highest recognition given. The Platinum grade is significant not only because it places us in the top one percent of the over 100,000 companies evaluated by EcoVadis but also represents a step up from the Gold Rating we received the previous year. We view this achievement as a testament to our continuous efforts and dedication to ESG management from a variety of perspectives. We published annual ESG reports that outline our green de-carbonization strategies and efforts to engage with suppliers to minimize potential social and ethical risks. In addition, Samsung Biologics is a core member of the Sustainable Markets Initiative (SMI) and support various global initiatives, including United Nations Global Compact (UNGC). Furthermore, we also became the first company in the Korean biopharma industry to have received the Leadership status and A- rating in the Climate Change category from the Carbon Disclosure project (CDP). The CDP evaluation is an indicator of a company's ability to respond to climate change, and investors and global pharma companies regard it as a reputable sustainability measure along with the Dow Jones Sustainability Index (DJSI). This is especially meaningful not only because it is the first time we attained this rating since participating in CDP in 2021, but also because we now are able to share the distinction alongside other major global pharma companies including AstraZeneca, GSK, J&J, and Sanofi, in recognition of our exceptional ability to respond to climate change. Samsung Biologics is committed to delivering sustainable value to our investors and stakeholders through environmental stewardship, social responsibility, and governance excellence. In closing, we would like to emphasize that we are committed to continually working to establish a sustainable future by balancing business growth and societal value creation as a leading CDMO. Your unwavering encouragement and support as we strive to lead by example as a top-tier biopharma company is much appreciated. Sincerely,
April 24, 2024 President & CEO, Samsung Biologics John Rim
CEO IR NEWSLETTER Driven. For Life.
SAMSUNG BIOLOGICS 2024.04
Dear valued investors and shareholders, This is John Rim from Samsung Biologics. First, I would like to express my gratitude for your continued interest and support this year.
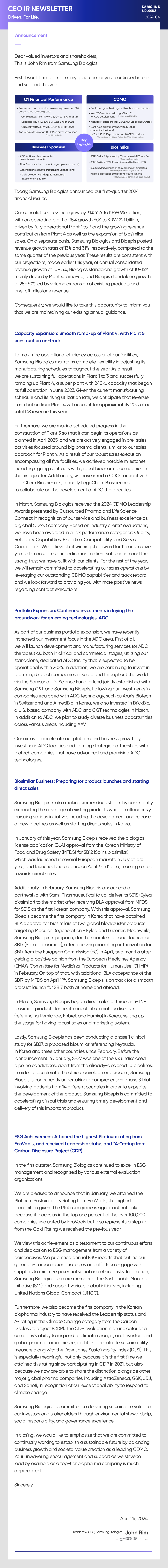
Key Highlights
- Q1 Financial Performance
- . P4 ramp-up and biosimilar business expansion led 31% consolidated revenue growth -Consolidated: Rev. KRW 947 B, OP. 221 B (OPM: 23.4%) -Separate: Rev. KRW 670 B, OP. 233 B (OPM: 34.8%) -Cumulative: Rev. KRW 280 B, OP. 38 B (OPM: 13.6%) . Annual sales to grow at 10 - 15% as previously guided * Consolidated basis
- CDMO
- . Continued growth with global biopharma companies . New CDO contract with LigaChem Bio *Former LegoChem Bio . Won all six categories for '24 CDMO Leadership Awards . Continued order momentum: USD 12.5 B contract value (cum.) - Total 90 CMO products and 116 CDO products * Secured one additional Global Top 20 Big Pharma client
- Business Expansion
- . ADC facility under construction (target operation within '24) . Plant 5 construction on-track (target operation in Apr. '25) . Continued investments through Life Science Fund: - Collaboration with Flagship Pioneering - Investment in BrickBio
- Biosimilar
- . SB17(bStelara): Approved by EC and Korea MFDS (Apr. '24) *European Commission . SB12(bSoliris) / SB15(bEylea): Approved by Korea MFDS . SB27(bKeytruda): Initiation of global phase 1 clinical trial * Concurrent phase 3 trial began in Apr. '24 . Initiated direct sales of three key products in Korea *SB2[bRemicade), SB4(bEnbrel), SB5(bHumira)
Today, Samsung Biologics announced our first-quarter 2024 financial results. Our consolidated revenue grew by 31% YoY to KRW 947 billion, with an operating profit of 15% growth YoY to KRW 221 billion, driven by fully operational Plant 1 to 3 and the growing revenue contribution from Plant 4 as well as the expansion of biosimilar sales. On a separate basis, Samsung Biologics and Bioepis posted revenue growth rates of 13% and 31%, respectively, compared to the same quarter of the previous year. These results are consistent with our projections, made earlier this year, of annual consolidated revenue growth of 10-15%, Biologics standalone growth of 10-15% mainly driven by Plant 4 ramp-up, and Bioepis standalone growth of 25-30% led by volume expansion of existing products and one-off milestone revenue. Consequently, we would like to take this opportunity to inform you that we are maintaining our existing annual guidance.
Capacity Expansion: Smooth ramp-up of Plant 4, with Plant 5 construction on-track
To maximize operational efficiency across all of our facilities, Samsung Biologics maintains complete flexibility in adjusting its manufacturing schedules throughout the year. As a result, we are sustaining full operations in Plant 1 to 3 and successfully ramping up Plant 4, a super plant with 240kL capacity that began its full operation in June 2023. Given the current manufacturing schedule and its rising utilization rate, we anticipate that revenue contribution from Plant 4 will account for approximately 20% of our total DS revenue this year. Furthermore, we are making scheduled progress in the construction of Plant 5 so that it can begin its operations as planned in April 2025, and we are actively engaged in pre-sales activities focused around big pharma clients, similar to our sales approach for Plant 4. As a result of our robust sales execution encompassing all five facilities, we achieved notable milestones including signing contracts with global biopharma companies in the first quarter. Additionally, we have inked a CDO contract with LigaChem Biosciences, formerly LegoChem Biosciences, to collaborate on the development of ADC therapeutics. In March, Samsung Biologics received the 2024 CDMO Leadership Awards presented by Outsourced Pharma and Life Science Connect in recognition of our service and business excellence as a global CDMO company. Based on industry clients' evaluations, we have been awarded in all six performance categories: Quality, Reliability, Capabilities, Expertise, Compatibility, and Service Capabilities. We believe that winning the award for 11 consecutive years demonstrates our dedication to client satisfaction and the strong trust we have built with our clients. For the rest of the year, we will remain committed to accelerating our sales operations by leveraging our outstanding CDMO capabilities and track record, and we look forward to providing you with more positive news regarding contract executions.
Portfolio Expansion: Continued investments in laying the groundwork for emerging technologies, ADC
As part of our business portfolio expansion, we have recently increased our investment focus in the ADC area. First of all, we will launch development and manufacturing services for ADC therapeutics, both in clinical and commercial stages, utilizing our standalone, dedicated ADC facility that is expected to be operational within 2024. In addition, we are continuing to invest in promising biotech companies in Korea and throughout the world via the Samsung Life Science Fund, a fund jointly established with Samsung C&T and Samsung Bioepis. Following our investments in companies equipped with ADC technology, such as Araris Biotech in Switzerland and AimedBio in Korea, we also invested in BrickBio, a U.S. based company with ADC and CGT technologies in March. In addition to ADC, we plan to study diverse business opportunities across various areas including AAV. Our aim is to accelerate our platform and business growth by investing in ADC facilities and forming strategic partnerships with biotech companies that have advanced and promising ADC technologies.
Biosimilar Business: Preparing for product launches and starting direct sales
Samsung Bioepis is also making tremendous strides by consistently expanding the coverage of existing products while simultaneously pursuing various initiatives including the development and release of new pipelines as well as starting directs sales in Korea. In January of this year, Samsung Bioepis received the biologics license application (BLA) approval from the Korean Ministry of Food and Drug Safety (MFDS) for SB12 (Soliris biosimilar), which was launched in several European markets in July of last year, and launched the product on April 1st in Korea, marking a step towards direct sales. Additionally, in February, Samsung Bioepis announced a partnership with Samil Pharmaceutical to co-deliver its SB15 (Eylea biosimilar) to the market after receiving BLA approval from MFDS for SB15 as the first Korean company. With this approval, Samsung Bioepis became the first company in Korea that have obtained BLA approval for biosimilars of two global blockbuster products targeting Macular Degeneration - Eylea and Lucentis. Meanwhile, Samsung Bioepis is preparing for the seamless product launch for SB17 (Stelara biosimilar), after receiving marketing authorization for SB17 from the European Commission (EC) in April, two months after getting a positive opinion from the European Medicines Agency (EMA)'s Committee for Medicinal Products for Human Use (CHMP) in February. On top of that, with additional BLA acceptance of the SB17 by MFDS on April 11th, Samsung Bioepis is on track for a smooth product launch for SB17 both at home and abroad. In March, Samsung Bioepis began direct sales of three anti-TNF biosimilar products for treatment of inflammatory diseases (referencing Remicade, Enbrel, and Humira) in Korea, setting up the stage for having robust sales and marketing system. Lastly, Samsung Bioepis has been conducting a phase 1 clinical study for SB27, a proposed biosimilar referencing Keytruda, in Korea and three other countries since February. Before the announcement in January, SB27 was one of the six undisclosed pipeline candidates, apart from the already-disclosed 10 pipelines. In order to accelerate the clinical development process, Samsung Bioepis is concurrently undertaking a comprehensive phase 3 trial involving patients from 14 different countries in order to expedite the development of the product. Samsung Bioepis is committed to accelerating clinical trials and ensuring timely development and delivery of this important product.
ESG Achievement: Attained the highest Platinum rating from EcoVadis, and received Leadership status and "A-"rating from Carbon Disclosure Project (CDP)
In the first quarter, Samsung Biologics continued to excel in ESG management and recognized by various external evaluation organizations. We are pleased to announce that in January, we attained the Platinum Sustainability Rating from EcoVadis, the highest recognition given. The Platinum grade is significant not only because it places us in the top one percent of the over 100,000 companies evaluated by EcoVadis but also represents a step up from the Gold Rating we received the previous year. We view this achievement as a testament to our continuous efforts and dedication to ESG management from a variety of perspectives. We published annual ESG reports that outline our green de-carbonization strategies and efforts to engage with suppliers to minimize potential social and ethical risks. In addition, Samsung Biologics is a core member of the Sustainable Markets Initiative (SMI) and support various global initiatives, including United Nations Global Compact (UNGC). Furthermore, we also became the first company in the Korean biopharma industry to have received the Leadership status and A- rating in the Climate Change category from the Carbon Disclosure project (CDP). The CDP evaluation is an indicator of a company's ability to respond to climate change, and investors and global pharma companies regard it as a reputable sustainability measure along with the Dow Jones Sustainability Index (DJSI). This is especially meaningful not only because it is the first time we attained this rating since participating in CDP in 2021, but also because we now are able to share the distinction alongside other major global pharma companies including AstraZeneca, GSK, J&J, and Sanofi, in recognition of our exceptional ability to respond to climate change. Samsung Biologics is committed to delivering sustainable value to our investors and stakeholders through environmental stewardship, social responsibility, and governance excellence. In closing, we would like to emphasize that we are committed to continually working to establish a sustainable future by balancing business growth and societal value creation as a leading CDMO. Your unwavering encouragement and support as we strive to lead by example as a top-tier biopharma company is much appreciated. Sincerely,
April 24, 2024 President & CEO, Samsung Biologics John Rim
- CDO
- CGMP
- ADC
- Bio Campus
- IR
- CMO