

Dear Investors, This is John Rim, CEO of Samsung Biologics.
I would like to express my deep gratitude and appreciation for your continued interest and support in Samsung Biologics.
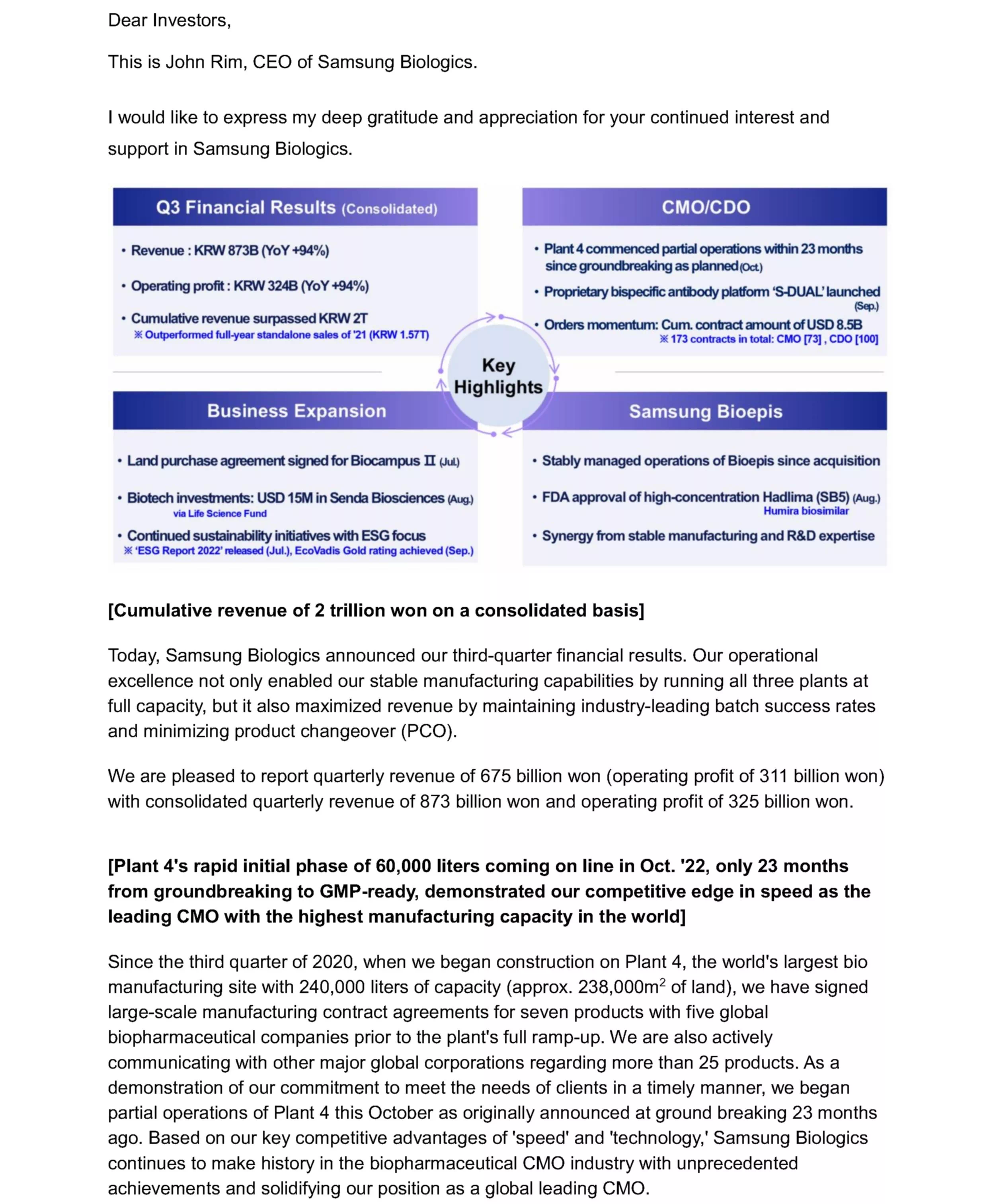
- Q3 Financial Results(consolidated)
Revenue : KRW873B(YoY +94%)
Operating profit : KRW 324B(YoY +94%)
Cumulative revenue surpassed KRW 2T *Outperformed full-year standalone sales of '21(KRW 1.57T)
- CMO/CDO
Plant4 commenced partial operations within 23 months since groundbreaking as planned(Oct.)
Proprietary bispecific antibody platform 'S-DUAL'launched(Sep.)
Orders momentum: Cum. contract amount of USD 8.5B *173 contracts in total : CMO [73], CDO[100]
- Business Expansion
Land purchase agreement signed for Biocampus 2(Jul)
Biotech investments: USD 15M in Senda Biosciences(Aug.) via Life Science Fund
Continued sustainability initiatives with ESG focus * ESG Report 2022 released(Jul.), Ecovadis Gold rating achieved(Sep.)
- Samsung Bioepis
Stably managed operations of Bioepis since acquisition
FDA approval of high-concentration Hadllima (SB5) (Aug.) Humira biosimilar
Synergy from stable manufacturing and R&D expertise
[cumulative revenue of 2 trillion won on a consolidated basis]
Today, Samsung Biologics announced our third-quarter financial results. Our operational excellence not only enabled our stable manufacturing capabilities by renning all three plants at full capacity, but it also mximized revenue by maintaining industry-leading batch success rates and minimizing product changeover(PCO).
We are pleased to report quarterly revenue of 675 billion won(operating profit of 311 billion won) with consolidate quarterly revenue of 873 billion won and operating profit of 325 billion won.
[Plant 4's rapid initial phase of 60,000 liters coming on line in Oct. '22, only 23 months from groundbreaking to GMP-ready, demonstrated our competitive edge in speed as the leading CMO with the highest manufacturing capacity in the world']
Since the third dquarter of 2020, when we began construction on Plant 4, the world's largest bio manufacturing site with 240,000 liters of capacity (approx. 238,000m2 of land), we have signed large-scale manufacturing contract agreements for seven products with five global biopharmaceutical companies prior to the plant's full ramp-up. We are also actively communicating with other major global corporations regarding more than 25 products. As a demonstration of our commitment to meet the needs of clients in a timely manner, we began partial operations of Plant 4 this October as originally announced at ground breaking 23 months ago. Based on our key competitive advantages of 'speed' and 'technology,' Samsung Biologics continues to make history in the biopharmaceutical CMO industry with unprecedented achievements and solidifying our position as a global leading CMO.
[Expanding and diversifying our path to become the world's best CDMO]
In response to the increasing biopharmaceutical CMO demand, Samsung Biologics signed a purchase agreement with Incheon Metropolitan City in July to acquire an additional parcel of land (357,000m2) approximately 30% larger than our current campus (238,000m2). In addition, we have been expanding our CMO production facilities beyond monoclonal antibodies to accommodate a wide range of industry needs. For example, after 7 months of technology transfer and scale-up, we successfully completed our first commercial scale engineering run of an mRNA vaccine drug substance at our newly-built mRNA manufacturing suite at our Songdo headquarters. With this, we are now fully equipped to provide one-stop, end-to-end services for mRNA vaccines in addition to our flagship CMO services for antibody drugs.
Furthermore, Samsung Biologics has been steadily introducing a number of proprietary technology platforms, laying a solid foundation for our CDO business. Since entering the CDO market in 2018, we have launched a proprietary cell line expression technology called 'S-CHOice' in 2020. We also demonstrated our steady efforts this year by introducing two new platforms: a rapid developability assessment platform called 'DEVELOPICKTM,' which helps identify candidates with the best potential for IND and BLA at an early development stage, and a bispecific antibody platform called 'S-DUALTM,' which ensures optimized manufacturability of bispecific antibodies. In addition to our solid track record and various self-developed platforms, including 'S-DUAL,' we plan to expand our partnership with R&D-based pharmaceutical companies to improve our capability in the CDO business and become a fully integrated, leading end-to-end CDMO.
Samsung Biologics' interests and efforts go beyond investments in existing businesses. In fact, we made our first investment in Jaguar Gene Therapy, a US biotech, through the Samsung Life Science Fund, a joint venture established with Samsung C&T, in March this year, followed in August by a $15M investment into a US therapeutics platform company named Senda Biosciences, which specializes in leveraging nanoparticles to deliver protein and peptide therapies. We will continue to invest in innovative venture firms both domestically and abroad to promote and nurture promising core bio-technologies for a brighter long-term future and growth of the biopharmaceutical industry.
[Leading ESG CDMO committed to sustainable growth, the environment, and society]
As always, Samsung Biologics continued our commitment to sustainable growth in the third quarter by implementing numerous ESG management initiatives to achieve tangible outcomes. In September, Samsung Biologics received a 'Gold' rating from Ecovadis, a leading global evaluator for sustainable CSR performance in recognition of our ongoing sustainable management efforts as outlined in our ESG Report 2022 (published in July), and joined the top 5% of the more than 100,000 companies worldwide assessed by the institution.
Samsung Biologics has also been an active participant in The Sustainable Markets Initiative Health Systems Task Force, along with other global industry leaders, to accelerate the delivery of industry-wide net zero across healthcare supply chains. By prioritizing sustainability commitments in our business while developing cutting-edge technologies and services that are adaptable to ever-changing market trends and dynamics, Samsung Biologics will strive to fulfill
our corporate responsibility and address the needs and expectations of all stakeholders and the broader society.
Thank you again for all your inspiration and encouragement. Please remember that your continuous support and trust in us will always be highly appreciated.
Sincerely,
October 26, 2022
John Rim
President & CEO, Samsung Biologics



Dear Investors, This is John Rim, CEO of Samsung Biologics.
I would like to express my deep gratitude and appreciation for your continued interest and support in Samsung Biologics.
- Q3 Financial Results(consolidated)
Revenue : KRW873B(YoY +94%)
Operating profit : KRW 324B(YoY +94%)
Cumulative revenue surpassed KRW 2T *Outperformed full-year standalone sales of '21(KRW 1.57T)
- CMO/CDO
Plant4 commenced partial operations within 23 months since groundbreaking as planned(Oct.)
Proprietary bispecific antibody platform 'S-DUAL'launched(Sep.)
Orders momentum: Cum. contract amount of USD 8.5B *173 contracts in total : CMO [73], CDO[100]
- Business Expansion
Land purchase agreement signed for Biocampus 2(Jul)
Biotech investments: USD 15M in Senda Biosciences(Aug.) via Life Science Fund
Continued sustainability initiatives with ESG focus * ESG Report 2022 released(Jul.), Ecovadis Gold rating achieved(Sep.)
- Samsung Bioepis
Stably managed operations of Bioepis since acquisition
FDA approval of high-concentration Hadllima (SB5) (Aug.) Humira biosimilar
Synergy from stable manufacturing and R&D expertise
[cumulative revenue of 2 trillion won on a consolidated basis]
Today, Samsung Biologics announced our third-quarter financial results. Our operational excellence not only enabled our stable manufacturing capabilities by renning all three plants at full capacity, but it also mximized revenue by maintaining industry-leading batch success rates and minimizing product changeover(PCO).
We are pleased to report quarterly revenue of 675 billion won(operating profit of 311 billion won) with consolidate quarterly revenue of 873 billion won and operating profit of 325 billion won.
[Plant 4's rapid initial phase of 60,000 liters coming on line in Oct. '22, only 23 months from groundbreaking to GMP-ready, demonstrated our competitive edge in speed as the leading CMO with the highest manufacturing capacity in the world']
Since the third dquarter of 2020, when we began construction on Plant 4, the world's largest bio manufacturing site with 240,000 liters of capacity (approx. 238,000m2 of land), we have signed large-scale manufacturing contract agreements for seven products with five global biopharmaceutical companies prior to the plant's full ramp-up. We are also actively communicating with other major global corporations regarding more than 25 products. As a demonstration of our commitment to meet the needs of clients in a timely manner, we began partial operations of Plant 4 this October as originally announced at ground breaking 23 months ago. Based on our key competitive advantages of 'speed' and 'technology,' Samsung Biologics continues to make history in the biopharmaceutical CMO industry with unprecedented achievements and solidifying our position as a global leading CMO.
[Expanding and diversifying our path to become the world's best CDMO]
In response to the increasing biopharmaceutical CMO demand, Samsung Biologics signed a purchase agreement with Incheon Metropolitan City in July to acquire an additional parcel of land (357,000m2) approximately 30% larger than our current campus (238,000m2). In addition, we have been expanding our CMO production facilities beyond monoclonal antibodies to accommodate a wide range of industry needs. For example, after 7 months of technology transfer and scale-up, we successfully completed our first commercial scale engineering run of an mRNA vaccine drug substance at our newly-built mRNA manufacturing suite at our Songdo headquarters. With this, we are now fully equipped to provide one-stop, end-to-end services for mRNA vaccines in addition to our flagship CMO services for antibody drugs.
Furthermore, Samsung Biologics has been steadily introducing a number of proprietary technology platforms, laying a solid foundation for our CDO business. Since entering the CDO market in 2018, we have launched a proprietary cell line expression technology called 'S-CHOice' in 2020. We also demonstrated our steady efforts this year by introducing two new platforms: a rapid developability assessment platform called 'DEVELOPICKTM,' which helps identify candidates with the best potential for IND and BLA at an early development stage, and a bispecific antibody platform called 'S-DUALTM,' which ensures optimized manufacturability of bispecific antibodies. In addition to our solid track record and various self-developed platforms, including 'S-DUAL,' we plan to expand our partnership with R&D-based pharmaceutical companies to improve our capability in the CDO business and become a fully integrated, leading end-to-end CDMO.
Samsung Biologics' interests and efforts go beyond investments in existing businesses. In fact, we made our first investment in Jaguar Gene Therapy, a US biotech, through the Samsung Life Science Fund, a joint venture established with Samsung C&T, in March this year, followed in August by a $15M investment into a US therapeutics platform company named Senda Biosciences, which specializes in leveraging nanoparticles to deliver protein and peptide therapies. We will continue to invest in innovative venture firms both domestically and abroad to promote and nurture promising core bio-technologies for a brighter long-term future and growth of the biopharmaceutical industry.
[Leading ESG CDMO committed to sustainable growth, the environment, and society]
As always, Samsung Biologics continued our commitment to sustainable growth in the third quarter by implementing numerous ESG management initiatives to achieve tangible outcomes. In September, Samsung Biologics received a 'Gold' rating from Ecovadis, a leading global evaluator for sustainable CSR performance in recognition of our ongoing sustainable management efforts as outlined in our ESG Report 2022 (published in July), and joined the top 5% of the more than 100,000 companies worldwide assessed by the institution.
Samsung Biologics has also been an active participant in The Sustainable Markets Initiative Health Systems Task Force, along with other global industry leaders, to accelerate the delivery of industry-wide net zero across healthcare supply chains. By prioritizing sustainability commitments in our business while developing cutting-edge technologies and services that are adaptable to ever-changing market trends and dynamics, Samsung Biologics will strive to fulfill
our corporate responsibility and address the needs and expectations of all stakeholders and the broader society.
Thank you again for all your inspiration and encouragement. Please remember that your continuous support and trust in us will always be highly appreciated.
Sincerely,
October 26, 2022
John Rim
President & CEO, Samsung Biologics
- CDO
- CGMP
- ADC
- Bio Campus
- IR
- CMO