Press Releases
Agile. Flexible. Focused on You.

Given the complexities and unpredictable nature of drug development, agility and flexibility are key service elements that a growing number of drug developers look for when partnering with a CDMO. In this article, we explore what value Samsung Biologics’ development capabilities can offer and how solutions are tailored to meet the specific needs of clients.
“Our CDO clients are the innovators who develop treatments that are currently not available. We are committed to supporting those innovators by taking their molecule through the development journey successfully from the beginning. We care about and consider the business achievement they want when working with us.”
- Jahoon Kang, Vice President of CDO Development

“Samsung Biologics has the ability to react quickly in a planned, risk-based way, enabling the delivery of new medicines with the most efficient timelines, irrespective of the challenges faced during development. The company is also flexible in that it adapts and changes strategies based on what is best for the molecule to overcome unplanned obstacles and maximize the probability of success.”
- Daniel Buckley, Senior Director & Head of Ab Cell Purification Development
Samsung Biologics is committed to helping pharma and biotech companies achieve development milestones – ensuring that a program runs smoothly and timelines are met. Building on scientific and regulatory expertise, dedicated teamwork, and thorough planning, the company continues to adapt to innovation and utilizes advanced technology to assess risks and manage challenges without compromising quality.
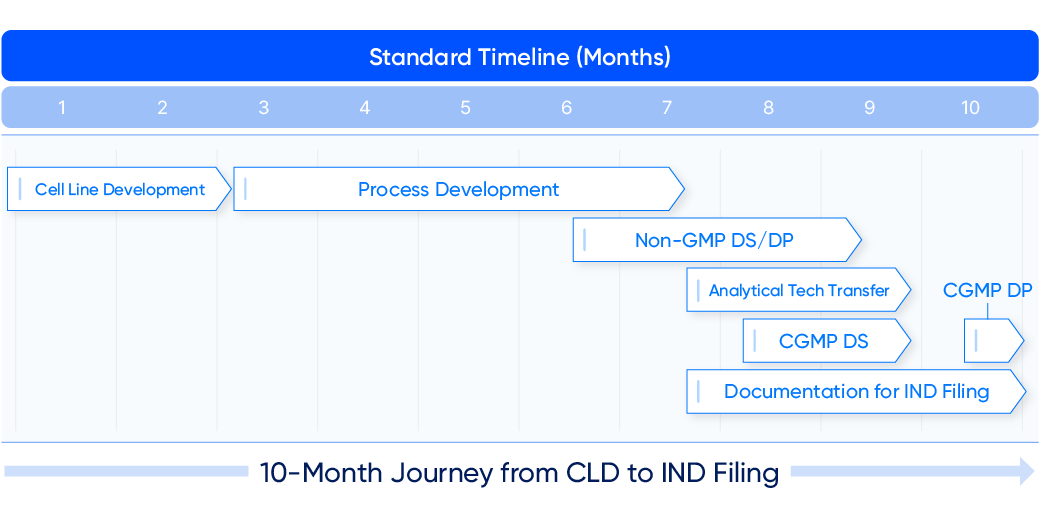
Standard Timeline* (Months)
- 1
Cell Line Development - 2
Cell Line Development - 3
Process Development - 4
Process Development - 5
Process Development - 6
Process Development
Non-GMP DS/DP - 7
Process Development
Non-GMP DS/DP
Analytical Tech Transfer
CGMP DS
Documentation for IND Filing - 8
Non-GMP DS/DP
Analytical Tech Transfer
CGMP DS
Documentation for IND Filing - 9
Analytical Tech Transfer
CGMP DS
Documentation for IND Filing - 10
CGMP DP
Documentation for IND Filing
10-Month Journey from CLD to IND Filing
Samsung Biologics offers a 10-month timeline from cell line development to Investigational New Drug (IND) filing for antibodies and 12 months for complex. The company has launched proprietary technology platforms that can assess molecules and increase ex-pression as well as enhance quality and accelerate timelines.
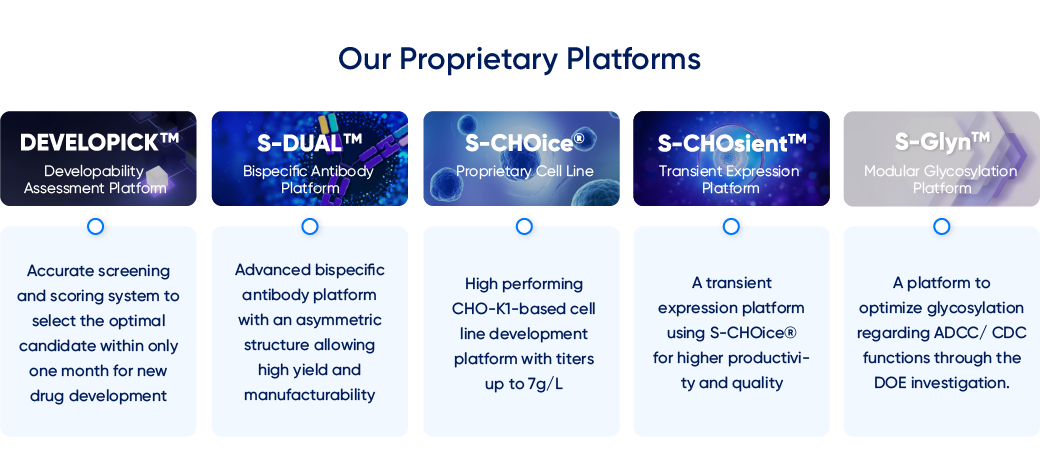
Our Proprietary Platforms
- DEVELOPICK™
Developability
Assessment Platform - Accurate screening and scoring system to select the optimal candidate within only one month for new drug development
- DEVELOPICK™
- S-DUAL™
Bispecific Antibody
Platform - Advanced bispecific antibody platform with an asymmetric structure allowing high yield and manufacturability
- S-DUAL™
- S-CHOice®
Proprietary Cell Line - High performing CHO-K1-based cell line development platform with titers up to 7g/L
- S-CHOice®
- S-CHOsient™
Transient Expression
Platform - A transient expression platform using S-CHOice® for higher productivi- ty and quality
- S-CHOsient™
- S-Glyn™
Modular Glycosylation
Platform - A platform to optimize glycosylation regarding ADCC/ CDC functions through the DOE investigation.
- S-Glyn™

“For every single project, our experts and scientists from each field come together to understand the unique characteristics of a client’s molecule. Based on our past development experience and after an in-depth analysis, we draw up a personalized solution depending on the client’s needs. During development, seamless communication and collaboration across teams has enabled us to promptly respond when any issue arises.”
- Suhil Chung, Senior Manager in CDO Support
Samsung Biologics also offers customized development services – SelecTailor ™ – whereby clients can choose from three packages that best fits their specific goals. Each package is designed to ensure maximum flexibility as clients can fine-tune their own programs by adding service options.
Utilizing pre-built modules, the company implements a highly streamlined approach, while assessing potential risks. This framework gives clients a guideline of where to start and what to expect – speeding up the process and allowing the implementation of appropriate strategies along the way.

- Simplified IND Package
- A compact service package that focuses on the Phase 1IND process utilizing Samsung Biologics' platform
- Comprehensive IND Package
- A risk-reduced service package, providing a multi-faceted viewpoint to bolster the strength of the pipeline
- Enhanced CMC Package
- A CMC replacement service package, providing changes that improve upon key features
Samsung Biologics also provides a seamless transition from development to clinical manufacturing and beyond, leveraging its track record of success in the contract manufacturing space – such as large capacity with bioreactors of multiple scale from 1,000 liters through 15,000 liters, GMP capabilities, global standard in tech transfer and raw materials procurement. The company also has vast experience in facility operations and regulatory inspections.

“As a trusted CDMO partner, we do our best to address continuously changing client needs during the course of development without any road bumps while providing full transparency to the clients along the way. Clients want us to step up in every aspect; they value our opinion in decision making when carrying out the program.”
- Jaewon Mun, Director in CDO Project Management
As competition intensifies to be the first to market, a robust development plan that maps out potential risks but commits to speed and quality from the initial stages is crucial. Samsung Biologics continues to optimize processes, train personnel, and expand its capacity with the utmost priority on client satisfaction.
The company understands that each molecule is unique, and our dedicated team can provide meticulous support at each stage of the journey to realize its full potential. Samsung Biologics is committed to delivering on our promises to clients: ensuring the success of their programs and ultimately, helping patients with unmet medical needs.
If you are a biotech seeking to outsource your project from cell line development to IND, we have extensive experience offering innovative solutions to clients. Read about our two recent partnership success stories below.

- Forging a trusted partnership in end-to-end drug development
- "Samsung Biologics quality-centric, speedy execution enabled us to achieve our project goal."

- Building a transformative partnership in drug development
- "Samsung's CLD service helped our program regain momentum."
To find out more about our development services and how we can meet your project deliverables, please visit: https://samsungbiologics.com/services/cdo/overview
Related Contents
Samsung BIO Insight Our All-in-One CDO Service Guide
Samsung BIO Insight Creating a reliable foundation for end-to-end drug development
Samsung BIO Insight Customized CMC Solutions: The best fit for your development strategy

Given the complexities and unpredictable nature of drug development, agility and flexibility are key service elements that a growing number of drug developers look for when partnering with a CDMO. In this article, we explore what value Samsung Biologics’ development capabilities can offer and how solutions are tailored to meet the specific needs of clients.
“Our CDO clients are the innovators who develop treatments that are currently not available. We are committed to supporting those innovators by taking their molecule through the development journey successfully from the beginning. We care about and consider the business achievement they want when working with us.”
- Jahoon Kang, Vice President of CDO Development

“Samsung Biologics has the ability to react quickly in a planned, risk-based way, enabling the delivery of new medicines with the most efficient timelines, irrespective of the challenges faced during development. The company is also flexible in that it adapts and changes strategies based on what is best for the molecule to overcome unplanned obstacles and maximize the probability of success.”
- Daniel Buckley, Senior Director & Head of Ab Cell Purification Development
Samsung Biologics is committed to helping pharma and biotech companies achieve development milestones – ensuring that a program runs smoothly and timelines are met. Building on scientific and regulatory expertise, dedicated teamwork, and thorough planning, the company continues to adapt to innovation and utilizes advanced technology to assess risks and manage challenges without compromising quality.

Samsung Biologics offers a 10-month timeline from cell line development to Investigational New Drug (IND) filing for antibodies and 12 months for complex. The company has launched proprietary technology platforms that can assess molecules and increase ex-pression as well as enhance quality and accelerate timelines.

Our Proprietary Platforms
- DEVELOPICK™
Developability
Assessment Platform - Accurate screening and scoring system to select the optimal candidate within only one month for new drug development
- DEVELOPICK™
- S-DUAL™
Bispecific Antibody
Platform - Advanced bispecific antibody platform with an asymmetric structure allowing high yield and manufacturability
- S-DUAL™
- S-CHOice®
Proprietary Cell Line - High performing CHO-K1-based cell line development platform with titers up to 7g/L
- S-CHOice®
- S-CHOsient™
Transient Expression
Platform - A transient expression platform using S-CHOice® for higher productivi- ty and quality
- S-CHOsient™
- S-Glyn™
Modular Glycosylation
Platform - A platform to optimize glycosylation regarding ADCC/ CDC functions through the DOE investigation.
- S-Glyn™

“For every single project, our experts and scientists from each field come together to understand the unique characteristics of a client’s molecule. Based on our past development experience and after an in-depth analysis, we draw up a personalized solution depending on the client’s needs. During development, seamless communication and collaboration across teams has enabled us to promptly respond when any issue arises.”
- Suhil Chung, Senior Manager in CDO Support
Samsung Biologics also offers customized development services – SelecTailor ™ – whereby clients can choose from three packages that best fits their specific goals. Each package is designed to ensure maximum flexibility as clients can fine-tune their own programs by adding service options.
Utilizing pre-built modules, the company implements a highly streamlined approach, while assessing potential risks. This framework gives clients a guideline of where to start and what to expect – speeding up the process and allowing the implementation of appropriate strategies along the way.

- Simplified IND Package
- A compact service package that focuses on the Phase 1IND process utilizing Samsung Biologics' platform
- Comprehensive IND Package
- A risk-reduced service package, providing a multi-faceted viewpoint to bolster the strength of the pipeline
- Enhanced CMC Package
- A CMC replacement service package, providing changes that improve upon key features
Samsung Biologics also provides a seamless transition from development to clinical manufacturing and beyond, leveraging its track record of success in the contract manufacturing space – such as large capacity with bioreactors of multiple scale from 1,000 liters through 15,000 liters, GMP capabilities, global standard in tech transfer and raw materials procurement. The company also has vast experience in facility operations and regulatory inspections.

“As a trusted CDMO partner, we do our best to address continuously changing client needs during the course of development without any road bumps while providing full transparency to the clients along the way. Clients want us to step up in every aspect; they value our opinion in decision making when carrying out the program.”
- Jaewon Mun, Director in CDO Project Management
As competition intensifies to be the first to market, a robust development plan that maps out potential risks but commits to speed and quality from the initial stages is crucial. Samsung Biologics continues to optimize processes, train personnel, and expand its capacity with the utmost priority on client satisfaction.
The company understands that each molecule is unique, and our dedicated team can provide meticulous support at each stage of the journey to realize its full potential. Samsung Biologics is committed to delivering on our promises to clients: ensuring the success of their programs and ultimately, helping patients with unmet medical needs.
If you are a biotech seeking to outsource your project from cell line development to IND, we have extensive experience offering innovative solutions to clients. Read about our two recent partnership success stories below.

- Forging a trusted partnership in end-to-end drug development
- "Samsung Biologics quality-centric, speedy execution enabled us to achieve our project goal."

- Building a transformative partnership in drug development
- "Samsung's CLD service helped our program regain momentum."
To find out more about our development services and how we can meet your project deliverables, please visit: https://samsungbiologics.com/services/cdo/overview
Related Contents
Samsung BIO Insight Our All-in-One CDO Service Guide
Samsung BIO Insight Creating a reliable foundation for end-to-end drug development
Samsung BIO Insight Customized CMC Solutions: The best fit for your development strategy