Customized CMC Solutions: The best fit for your development strategy
As competition intensifies and technology advances, molecules are becoming more complex with their unique characteristics - posing challenges and requiring longer timelines for development.
Having a robust development plan with well-thought-out strategies—one that considers potential risks but commits to speed and quality—right from the beginning is crucial to advancing a drug candidate throughout the entire development path successfully.
In this article, we delve into how Samsung Biologics can steer clients through the drug journey and unleash the full potential of their candidates via its customizable CMC* solutions tailored to the development goals of each client.
*Chemical, manufacturing and control (CMC) activities are essential to drug development to meet quality, safety, and efficacy standards. It includes the development of manufacturing processes, quality control tests, and stability studies.
Tailored to achieve a client’s end goal
While monoclonal antibodies continue to lead the biologics market, innovation has increased demand for other modalities, such as bispecific antibodies, fusion proteins, and antibody-drug conjugates.
Successful drug development relies on a thorough understanding of a molecule’s characteristics. Each development journey is unique and complex, while each client’s needs are diverse—meaning flexible and customized approaches are essential to pave the way to the clinic.
Building on its proven track record of success as a contract manufacturing and development provider, Samsung Biologics has optimized development services incorporating innovative processes and advanced platforms** to accommodate clients’ evolving needs - supporting their journey from gene to investigational new drug (IND) and/or beyond.
**S-CHOICE® is a high-performing CHO-K1-based cell line development that improves titers.
**S-DUAL™ is an advanced high-yield bispecific antibody platform that enables high binding affinity to produce higher titer and purity.
**DEVELOPICK™ provides selection guidance to predict candidates with the best developability to minimize time and cost and maximize efficiency.
To proactively satisfy and serve a client’s specific needs, Samsung Biologics has packaged its drug development services into three programs—each designed to ensure maximum flexibility and meet the client’s goals per their development stage. Utilizing pre-built modules, Samsung Biologics implements a highly streamlined approach, while assessing potential risks that may occur along the way. This framework gives clients a guideline of where to start and what to expect—speeding up the process and mapping out the strategies.
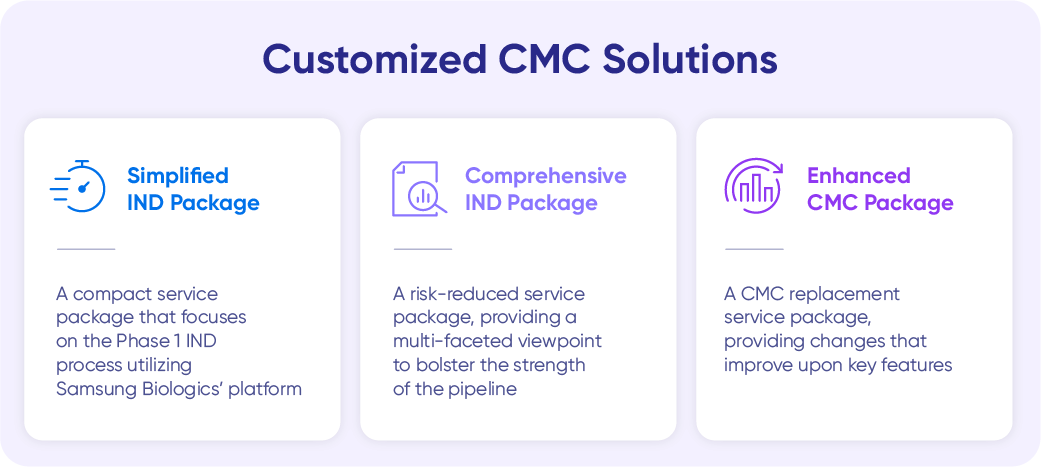
 Simplified IND Package
Simplified IND Package
The simplified IND package focuses on IND Phase I, utilizing Samsung Biologics’ proprietary platforms. The key priority is accelerating the timeline, while ensuring appropriate quality and effectiveness requirements. The company has designed the program to meet a timeline of 11 months for monoclonal antibodies and within 13 months for complex molecules.
 Comprehensive IND Package
Comprehensive IND Package
The comprehensive IND package provides a multi-faceted scope to clients who wish to mitigate risks and opt for a more stable development process. The service consists of robust data items, including CMC data and stability studies, while quality attributes beyond IND Phase I are assessed in advance to measure potential risks that may occur in the late phase. This program aims to support clients who want to take their pipelines to commercialization by pursuing a more detailed and comprehensive service with add-on options for complex mechanisms.
 Enhanced CMC Package
Enhanced CMC Package
The enhanced CMC package focuses on improving productivity. This program facilitates end-to-end provision through the replacement of cell lines, processes, or formulation to meet the client’s need for improvement and ensure a seamless journey to commercialization. Through this package, clients can leverage Samsung Biologics’ experience and expertise based on proven track records to enhance their manufacturability in need of improvement, while maintaining other quality attributes necessary to move on to the next development phase.
Each program—designed based on years of experience and expertise—comes with basic, indispensable requirements. Clients can also add a range of comprehensive service options to fine-tune their own best-fit package, depending on their development needs and molecule type.
A trusted CDMO partner to accelerate drug development success
As drug developers prioritize a first-to-market approach, a growing number of clients want to leverage a CDMO’s platform for faster development and their regulatory expertise to overcome unexpected challenges.
Samsung Biologics’ CDO services aim to achieve successful IND submission and minimize the chances of redevelopment. The company can also support a smooth and scalable manufacturing process with its world-class production capacity.

By utilizing cutting-edge technologies and innovative approaches, Samsung Biologics offers comprehensive strategies for both early and late stages from the beginning, resulting in streamlined development time and costs. This, in turn, helps clients expedite their development success and deliver life-saving biomedicines to patients worldwide.
Take the right step to advance your molecule from the beginning with Samsung Biologics. To find out more about our CDO capabilities, visit: https://samsungbiologics.com/services/cdo/overview
Related contents
Technology & Science Optimizing Drug Development Through Samsung Biologics' Customized CMC Solutions

As competition intensifies and technology advances, molecules are becoming more complex with their unique characteristics - posing challenges and requiring longer timelines for development.
Having a robust development plan with well-thought-out strategies—one that considers potential risks but commits to speed and quality—right from the beginning is crucial to advancing a drug candidate throughout the entire development path successfully.
In this article, we delve into how Samsung Biologics can steer clients through the drug journey and unleash the full potential of their candidates via its customizable CMC* solutions tailored to the development goals of each client.
*Chemical, manufacturing and control (CMC) activities are essential to drug development to meet quality, safety, and efficacy standards. It includes the development of manufacturing processes, quality control tests, and stability studies.
Tailored to achieve a client’s end goal
While monoclonal antibodies continue to lead the biologics market, innovation has increased demand for other modalities, such as bispecific antibodies, fusion proteins, and antibody-drug conjugates.
Successful drug development relies on a thorough understanding of a molecule’s characteristics. Each development journey is unique and complex, while each client’s needs are diverse—meaning flexible and customized approaches are essential to pave the way to the clinic.
Building on its proven track record of success as a contract manufacturing and development provider, Samsung Biologics has optimized development services incorporating innovative processes and advanced platforms** to accommodate clients’ evolving needs - supporting their journey from gene to investigational new drug (IND) and/or beyond.
**S-CHOICE® is a high-performing CHO-K1-based cell line development that improves titers.
**S-DUAL™ is an advanced high-yield bispecific antibody platform that enables high binding affinity to produce higher titer and purity.
**DEVELOPICK™ provides selection guidance to predict candidates with the best developability to minimize time and cost and maximize efficiency.
To proactively satisfy and serve a client’s specific needs, Samsung Biologics has packaged its drug development services into three programs—each designed to ensure maximum flexibility and meet the client’s goals per their development stage. Utilizing pre-built modules, Samsung Biologics implements a highly streamlined approach, while assessing potential risks that may occur along the way. This framework gives clients a guideline of where to start and what to expect—speeding up the process and mapping out the strategies.

 Simplified IND Package
Simplified IND Package
The simplified IND package focuses on IND Phase I, utilizing Samsung Biologics’ proprietary platforms. The key priority is accelerating the timeline, while ensuring appropriate quality and effectiveness requirements. The company has designed the program to meet a timeline of 11 months for monoclonal antibodies and within 13 months for complex molecules.
 Comprehensive IND Package
Comprehensive IND Package
The comprehensive IND package provides a multi-faceted scope to clients who wish to mitigate risks and opt for a more stable development process. The service consists of robust data items, including CMC data and stability studies, while quality attributes beyond IND Phase I are assessed in advance to measure potential risks that may occur in the late phase. This program aims to support clients who want to take their pipelines to commercialization by pursuing a more detailed and comprehensive service with add-on options for complex mechanisms.
 Enhanced CMC Package
Enhanced CMC Package
The enhanced CMC package focuses on improving productivity. This program facilitates end-to-end provision through the replacement of cell lines, processes, or formulation to meet the client’s need for improvement and ensure a seamless journey to commercialization. Through this package, clients can leverage Samsung Biologics’ experience and expertise based on proven track records to enhance their manufacturability in need of improvement, while maintaining other quality attributes necessary to move on to the next development phase.
Each program—designed based on years of experience and expertise—comes with basic, indispensable requirements. Clients can also add a range of comprehensive service options to fine-tune their own best-fit package, depending on their development needs and molecule type.
A trusted CDMO partner to accelerate drug development success
As drug developers prioritize a first-to-market approach, a growing number of clients want to leverage a CDMO’s platform for faster development and their regulatory expertise to overcome unexpected challenges.
Samsung Biologics’ CDO services aim to achieve successful IND submission and minimize the chances of redevelopment. The company can also support a smooth and scalable manufacturing process with its world-class production capacity.

By utilizing cutting-edge technologies and innovative approaches, Samsung Biologics offers comprehensive strategies for both early and late stages from the beginning, resulting in streamlined development time and costs. This, in turn, helps clients expedite their development success and deliver life-saving biomedicines to patients worldwide.
Take the right step to advance your molecule from the beginning with Samsung Biologics. To find out more about our CDO capabilities, visit: https://samsungbiologics.com/services/cdo/overview
Related contents
Technology & Science Optimizing Drug Development Through Samsung Biologics' Customized CMCSolutions
Share article
Related Content