Samsung Biologics launches high-concentration formulation platform to accelerate high-dose drug development

- S-HiConTM is designed to maximize drug delivery and stability through high-concentration formulation
- The platform can address challenges associated with viscosity and achieve stable liquid formulation for over 200 mg/mL subcutaneous administration
Incheon, S. Korea, October 14, 2024 – Samsung Biologics (KRX: 207940.KS), a global contract development and manufacturing organization (CDMO), launched today a new high-concentration formulation platform to support the development and manufacturing of high-dose biopharmaceuticals.
S-HiConTM can proactively identify unintended pH changes, enhance formulation stability, and reduce viscosity to ensure efficacy and maximize drug delivery. Through optimization of pH, buffer species, and excipients, along with a preliminary ‘Concentration Gate Check’ process, the platform tests formulation feasibility in the initial stages to identify favorable candidates and minimize potential risks associated with high concentration development.
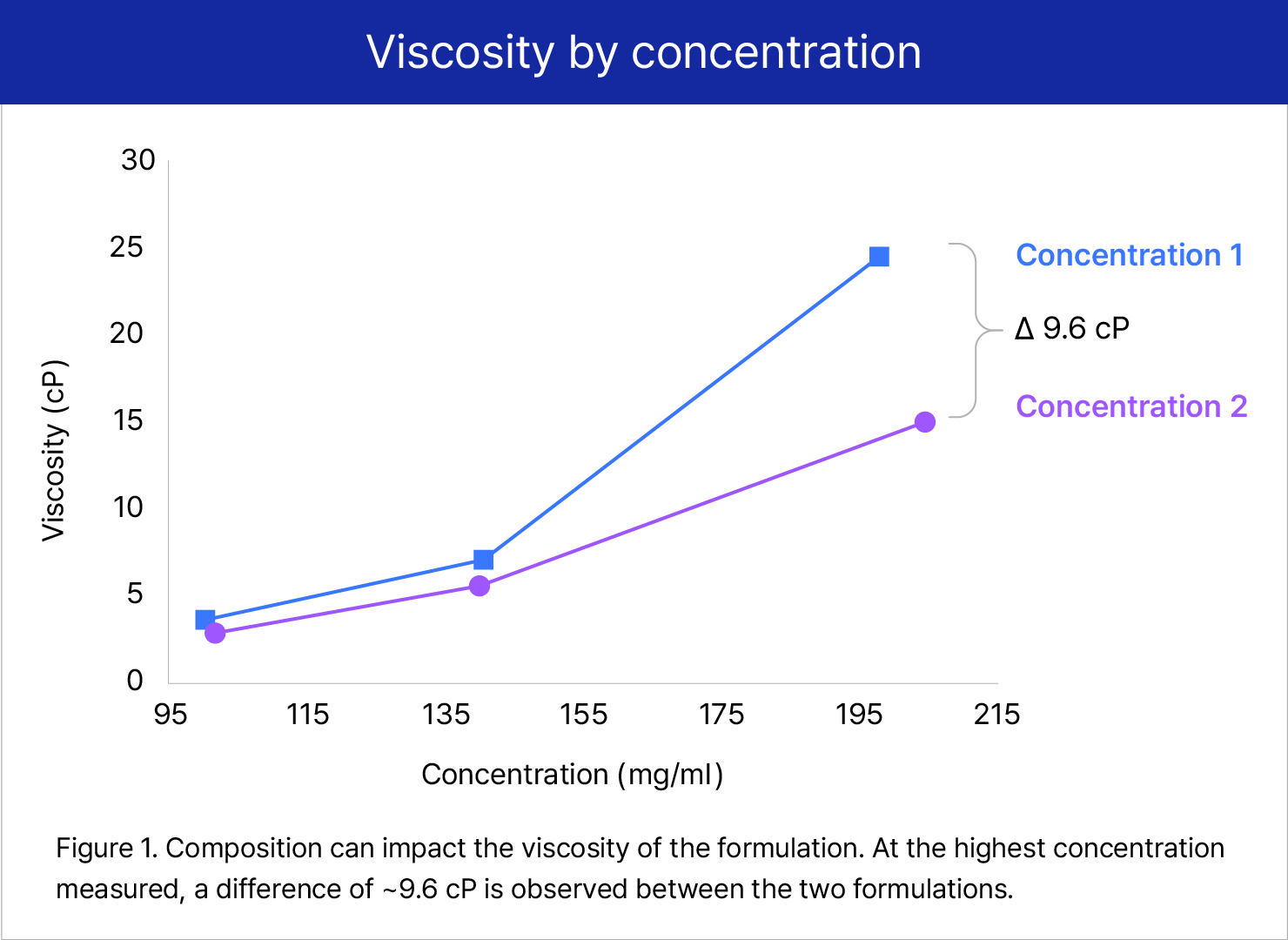
Additionally, S-HiConTM integrates analytical capabilities to ensure that critical formulation challenges associated with viscosity and protein aggregation are addressed (Figure 1). Samsung Biologics has recorded over 200 mg/mL for liquid formulations, with adequate viscosity and improved stability.
viscosity by concentration
Concentration 1, Concentration 2 9.6cP - Figure 1. Composition can impact the viscosity of the formulation. At the highest concentration measured, a difference of ~ 9.6 cP is observed between the two formulations.
“Our new platform will enable us to provide innovative solutions for clients requiring low to ultra-high concentration formulation to develop advanced therapeutics,” said Brian Hosung Min, Executive Vice President and Head of CDO Development. “Samsung Biologics is committed to providing customized services by leveraging our expertise and track record in contract development.”
The latest offering is part of Samsung Biologics’ continued efforts to provide clients with innovative technologies that enable high-quality development. Leveraging platforms that can increase antibody-dependent cellular cytotoxity (ADCC) activity and enhance upstream process quality, the company is expected to better accommodate clients’ evolving needs and support their pipelines. Visit our website to learn more about our CDO platforms: https://samsungbiologics.com/services/cdo/overview

- S-HiConTM is designed to maximize drug delivery and stability through high-concentration formulation
- The platform can address challenges associated with viscosity and achieve stable liquid formulation for over 200 mg/mL subcutaneous administration
Incheon, S. Korea, October 14, 2024 – Samsung Biologics (KRX: 207940.KS), a global contract development and manufacturing organization (CDMO), launched today a new high-concentration formulation platform to support the development and manufacturing of high-dose biopharmaceuticals.
S-HiConTM can proactively identify unintended pH changes, enhance formulation stability, and reduce viscosity to ensure efficacy and maximize drug delivery. Through optimization of pH, buffer species, and excipients, along with a preliminary ‘Concentration Gate Check’ process, the platform tests formulation feasibility in the initial stages to identify favorable candidates and minimize potential risks associated with high concentration development.
Additionally, S-HiConTM integrates analytical capabilities to ensure that critical formulation challenges associated with viscosity and protein aggregation are addressed (Figure 1). Samsung Biologics has recorded over 200 mg/mL for liquid formulations, with adequate viscosity and improved stability.

viscosity by concentration
Concentration 1, Concentration 2 9.6cP - Figure 1. Composition can impact the viscosity of the formulation. At the highest concentration measured, a difference of ~ 9.6 cP is observed between the two formulations.
“Our new platform will enable us to provide innovative solutions for clients requiring low to ultra-high concentration formulation to develop advanced therapeutics,” said Brian Hosung Min, Executive Vice President and Head of CDO Development. “Samsung Biologics is committed to providing customized services by leveraging our expertise and track record in contract development.”
The latest offering is part of Samsung Biologics’ continued efforts to provide clients with innovative technologies that enable high-quality development. Leveraging platforms that can increase antibody-dependent cellular cytotoxity (ADCC) activity and enhance upstream process quality, the company is expected to better accommodate clients’ evolving needs and support their pipelines. Visit our website to learn more about our CDO platforms: https://samsungbiologics.com/services/cdo/overview
Share article
Related Content