Press Releases
Samsung Biologics' all-in-one CMO service at a glance
Contract Manufacturing Service
Samsung Biologics' all-in-one CMO service at a glance
- How extensive is your contract manufacturing experience?
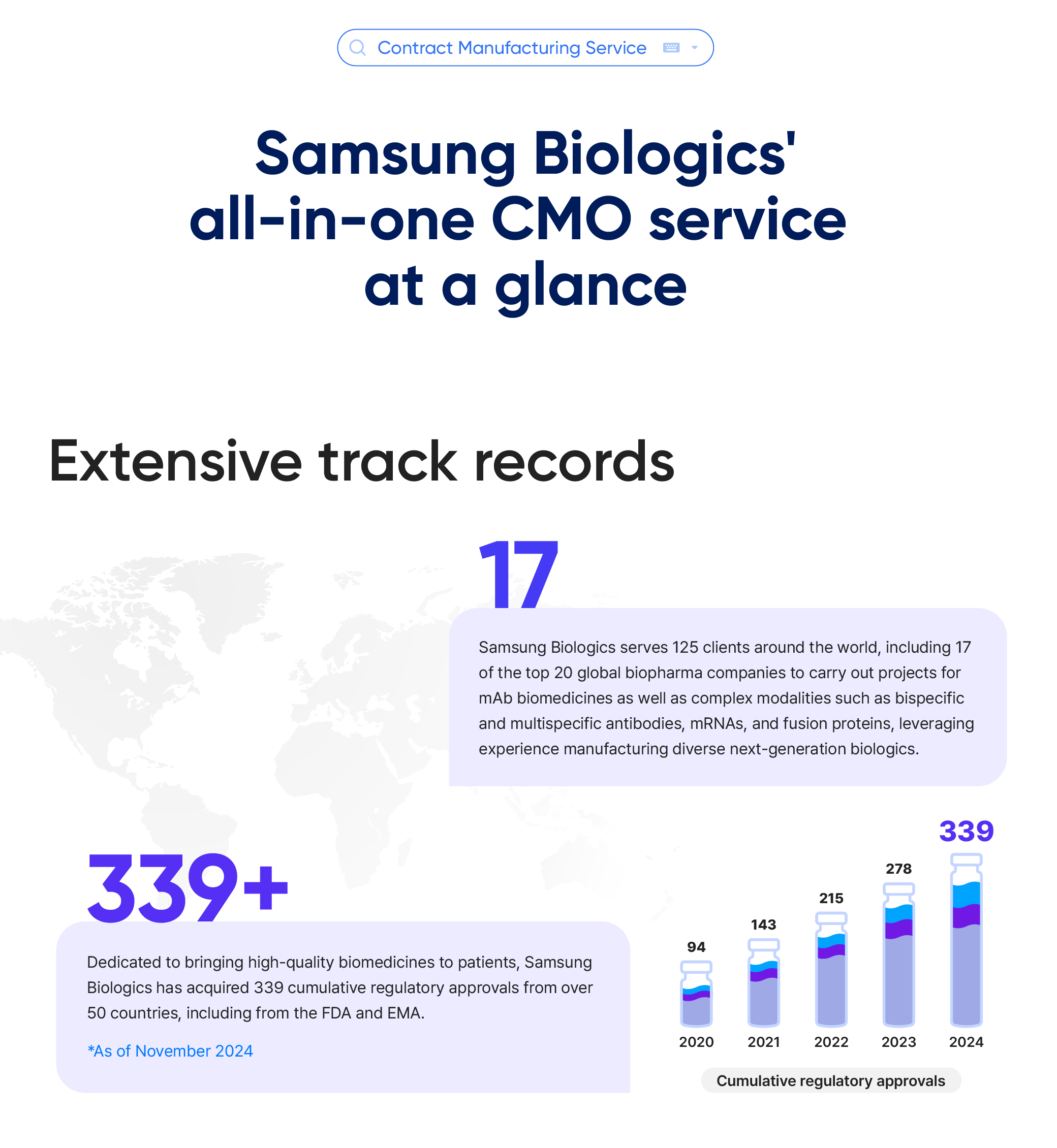
- 17 Samsung Biologics serves 125 clients around the world, including 17 of the top 20 global biopharma companies to carry out projects for mAb biomedicines as well as complex modalities such as bispecific and multispecific antibodies, mRNAs, and fusion proteins, leveraging experience manufacturing diverse next-generation biologics.
- 339+ Dedicated to bringing high-quality biomedicines to patients, Samsung Biologics has acquired 339 cumulative regulatory approvals from over 50 countries, including from the FDA and EMA. *As of November 2024
- Cumulative regulatory approvals
- 2020
- 94
- 2021
- 143
- 2022
- 215
- 2023
- 278
- 2024
- 339

- World's leading capacity and speed
784 KL Rapidly expanding market share while flexibly meeting changing demand. Through excellence and know-how in plant construction, Samsung Biologics is accelerating its capacity expansion to meet large-scale manufacturing demand. With Plant 5, which is planned for completion in April 2025, total capacity amounts to 784 KL.
Tech transfer & Operational efficiency One of Samsung Biologics' competitive advantages is the speed of tech transfer compared to the industry average. The CDMO has continuously reduced tech transfer time through process innovation, implementing continuous manufacturing via slowdown rather than shutdown of facility operations and enabling agile project timelines.
→ Learn more about our tech transfer capabilities
→ Learn more about our operational efficiency

- Proven quality
Batch Success Rate 98%
Samsung Biologics has recorded a batch success rate greater than 98%, building on collaboration with 125+ global biopharmaceutical companies and experience in what makes for successful projects. The CDMO is working to recruit quality experts to achieve an even greater batch success rate.
550+
Since the company's founding, a dedicated team for due diligence was formed to cultivate expertise, with professionals working toward global manufacturing approval now numbering over 550. Samsung Biologics is rapidly growing while practicing due diligence with clients and regulatory agencies more than 50 times a year.
SBL Client Portal
Sometimes numbers don't tell the whole story. Our 'Client Portal' offers convenience for clients, who can check the manufacturing status, process data, test results, and quality records for their products in real time.
- Data compliance
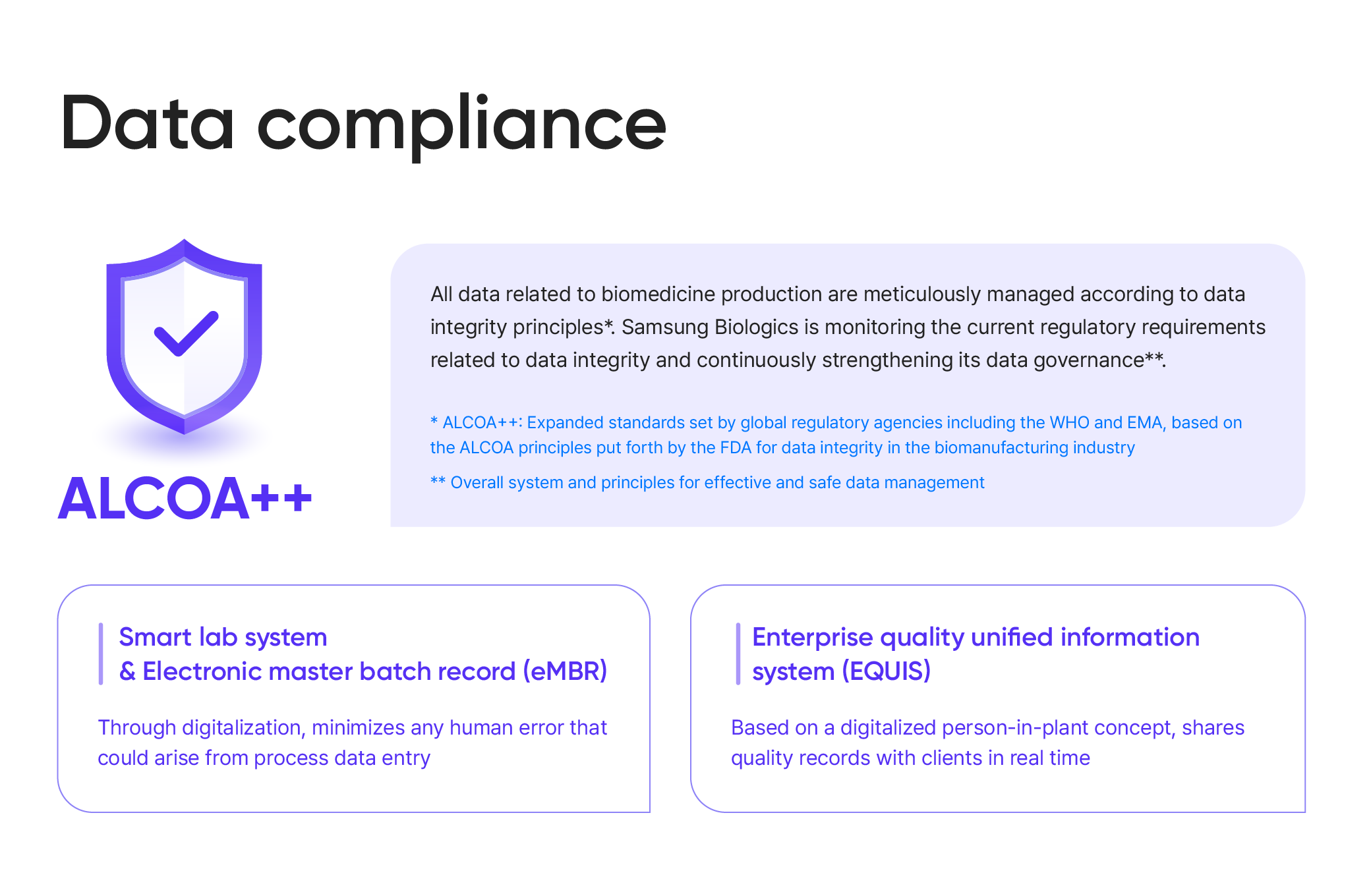
ALCOA++
All data related to biomedicine production are meticulously managed according to data integrity principles*. Samsung Biologics is monitoring the current regulatory requirements related to data integrity and continuously strengthening its data governance**
* ALCOA++: Expanded standards set by global regulatory agencies including the WHO and EMA, based on the ALCOA principles put forth by the FDA for data integrity in the biomanufacturing industry
** Overall system and principles for effective and safe data management
Smart lab system & Electronic master batch record (eMBR) Through digitalization, minimizes any human error that could arise from process data entry
Enterprise quality unified information system (EQUIS) Based on a digitalized person-in-plant concept, shares quality records with clients in real time
- Advanced technologies
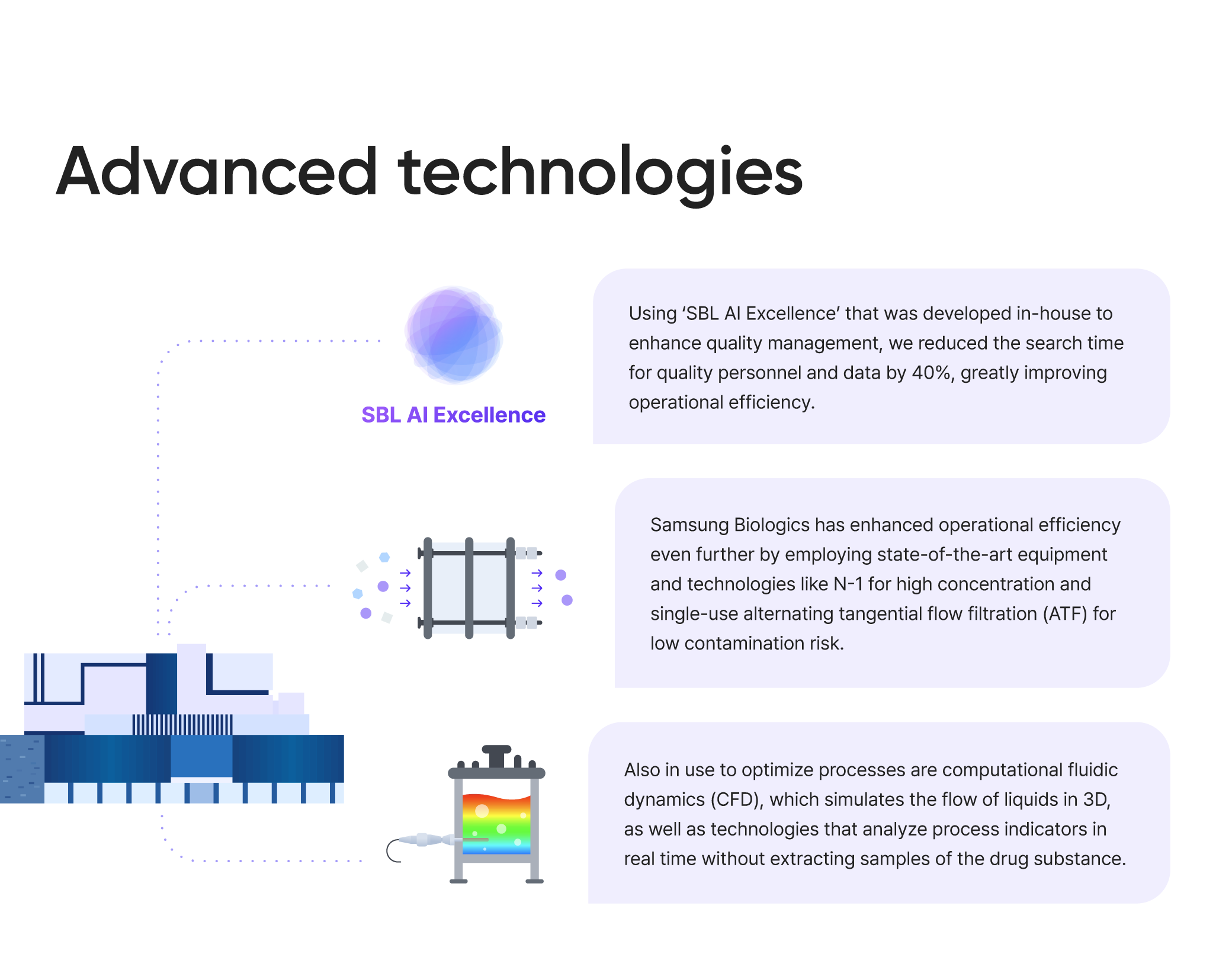
SBL AI Excellence
- Using 'SBL AI Excellence' that was developed in-house to enhance quality management, we reduced the search time for quality personnel and data by 40%, greatly improving operational efficiency.
- Samsung Biologics has enhanced operational efficiency even further by employing state-of-the art equipment and technologies like N-1 perfusion to reduce cell culture time and single-use alternating tangential flow filtration (ATF) to lower contamination risk.
- Also in use to optimize processes are computational fluidic dynamics (CFD), which simulates the flow of liquids in 3D, as well as technologies that analyze process indicators in real time without extracting samples of the drug substance.

- Continuous expansions
1,324 KL Samsung Biologics is to secure over 1.3 million liters of manufacturing capacity by 2032 through Bio Campus II. Also planned for completion by the end of 2024 is an antibody-drug conjugate (ADC) manufacturing facility of 500-L maximum capacity. Expansion plans into new modalities are in active discussion as well.
- Bio Campus I
- ADC manufacturing facility *Completion within 2024
- Plant 5 *Operational in April 2025
- Bio Campus II *Completion in 2032
Samsung Biologics is swiftly implementing new technologies and trends, securing facilities optimized for operational efficiency and current regulatory practices. By doing so, the CDMO steadily builds a partnership with its clients based on trust, backed by rich experience and manufacturing know-how.
→ Connect with oru manufacturing expert

Contract Manufacturing Service
Our all-in-one CMO service guide
- How extensive is your contract manufacturing experience?
- 17 Samsung Biologics serves 125 clients around the world, including 17 of the top 20 global biopharma companies to carry out projects for mAb biomedicines as well as complex modalities such as bispecific and multispecific antibodies, mRNAs, and fusion proteins, leveraging experience manufacturing diverse next-generation biologics.
- 339+ Dedicated to bringing high-quality biomedicines to patients, Samsung Biologics has acquired 339 cumulative regulatory approvals from over 50 countries, including from the FDA and EMA. *As of November 2024
- Cumulative regulatory approvals
- 2020
- 94
- 2021
- 143
- 2022
- 215
- 2023
- 278
- 2024
- 339

- World's leading capacity and speed
784 KL Rapidly expanding market share while flexibly meeting changing demand. Through excellence and know-how in plant construction, Samsung Biologics is accelerating its capacity expansion to meet large-scale manufacturing demand. With Plant 5, which is planned for completion in April 2025, total capacity amounts to 784 KL.
Tech transfer & Operational efficiency One of Samsung Biologics' competitive advantages is the speed of tech transfer compared to the industry average. The CDMO has continuously reduced tech transfer time through process innovation, implementing continuous manufacturing via slowdown rather than shutdown of facility operations and enabling agile project timelines.
→ Learn more about our tech transfer capabilities
→ Learn more about our operational efficiency

- Proven quality
550+
Since the company's founding, a dedicated team for due diligence was formed to cultivate expertise, with professionals working toward global manufacturing approval now numbering over 550. Samsung Biologics is rapidly growing while practicing due diligence with clients and regulatory agencies more than 50 times a year.
Batch Success Rate 98%
Samsung Biologics has recorded a batch success rate greater than 98%, building on collaboration with 125+ global biopharmaceutical companies and experience in what makes for successful projects. The CDMO is working to recruit quality experts to achieve an even greater batch success rate.
SBL Client Portal
Sometimes numbers don't tell the whole story. Our 'Client Portal' offers convenience for clients, who can check the manufacturing status, process data, test results, and quality records for their products in real time.

- Data compliance
ALCOA++
All data related to biomedicine production are meticulously managed according to data integrity principles*. Samsung Biologics is monitoring the current regulatory requirements related to data integrity and continuously strengthening its data governance**
* ALCOA++: Expanded standards set by global regulatory agencies including the WHO and EMA, based on the ALCOA principles put forth by the FDA for data integrity in the biomanufacturing industry
** Overall system and principles for effective and safe data management
Smart lab system & Electronic master batch record (eMBR) Through digitalization, minimizes any human error that could arise from process data entry
Enterprise quality unified information system (EQUIS) Based on a digitalized person-in-plant concept, shares quality records with clients in real time

- Advanced technologies
SBL AI Excellence
- Using 'SBL AI Excellence' that was developed in-house to enhance quality management, we reduced the search time for quality personnel and data by 40%, greatly improving operational efficiency.
- Samsung Biologics has enhanced operational efficiency even further by employing state-of-the art equipment and technologies like N-1 perfusion to reduce cell culture time and single-use alternating tangential flow filtration (ATF) to lower contamination risk.
- Also in use to optimize processes are computational fluidic dynamics (CFD), which simulates the flow of liquids in 3D, as well as technologies that analyze process indicators in real time without extracting samples of the drug substance.

- Continuous expansions
1,324 KL Samsung Biologics is to secure over 1.3 million liters of manufacturing capacity by 2032 through Bio Campus II. Also planned for completion by the end of 2024 is an antibody-drug conjugate (ADC) manufacturing facility of 500-L maximum capacity. Expansion plans into new modalities are in active discussion as well.
- Bio Campus I
- ADC manufacturing facility *Completion within 2024
- Plant 5 *Operational in April 2025
- Bio Campus II *Completion in 2032
Samsung Biologics is swiftly implementing new technologies and trends, securing facilities optimized for operational efficiency and current regulatory practices. By doing so, the CDMO steadily builds a partnership with its clients based on trust, backed by rich experience and manufacturing know-how.
→ Meet our manufacturing expert