
Biopharmaceutical manufacturing requires specialized facilities that meet strict regulatory standards to ensure product quality and patient safety. These facilities must be versatile enough to handle unique and complex molecules, including multispecific antibodies, antibody-drug conjugates, and mRNA therapies. Each product type requires highly compatible equipment, environmental controls, and manufacturing processes, adding complexity to facility design and construction.
Samsung Biologics aims to build flexible and efficient facilities to meet each client’s needs. “Our goal is to help our clients achieve faster time to market and reduce costs while maintaining the highest standards of quality,” says Junghyun Bak, Director of Engineering Planning at Samsung Biologics. “By using advanced engineering tools and leveraging our extensive experience, we streamline the entire facility development process.”
Proven track record and expertise in facility design and engineering
From years of experience, Samsung Biologics has refined its facility design methodology to minimize inefficiencies, reduce costs, and shorten project timelines. Our comprehensive strategy encompasses all facility development phases—from design and construction to operational readiness—ensuring each manufacturing site is ready to efficiently produce high-quality biopharmaceutical products.
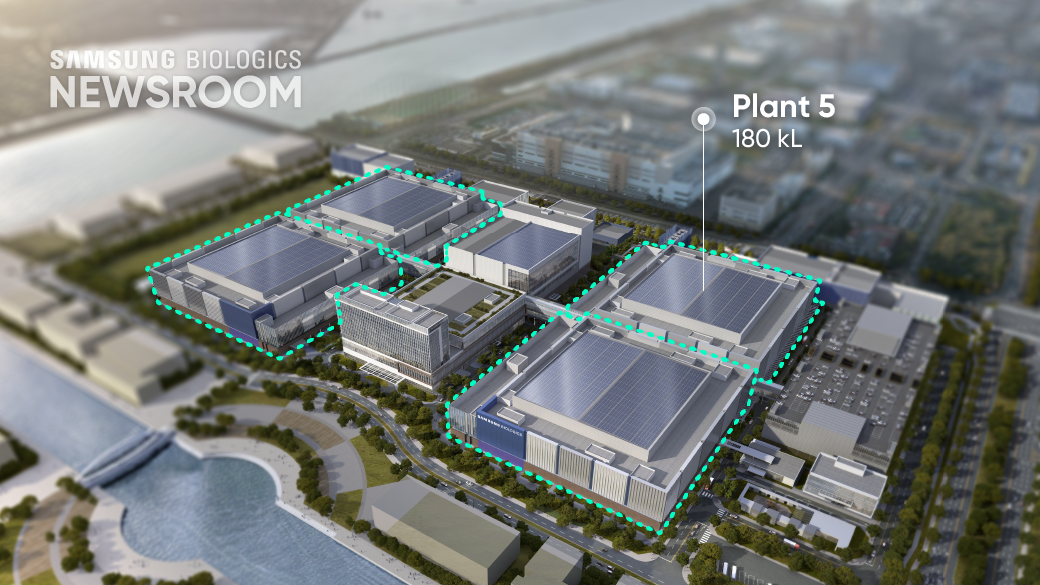
Samsung Biologics successfully completed four large-scale plants, demonstrating a strong ability to manage complex projects effectively. The company is currently building Plant 5, the first facility of Bio Campus II, whichwill be operational by April 2025. The construction timeline is expected to be approximately 30% shorter than the 35 months taken to complete Plant 3, which has the same capacity. The shortened timeline underscores the company’s expertise and commitment to expanding manufacturing capabilities to meet diverse and immediate client needs.

Advanced engineering and estimation tools are crucial for meticulously planning facility development, including material and utility requirements, project timelines, and cost estimates. Using these tools and a project management information system for real-time management, Samsung Biologics significantly reduced the risk of cost overruns and delays, allowing for efficient resource allocation, benefiting the company and our clients.
Samsung Biologics continuously improves facility design, construction, and validation methodologies. Each new plant implements best practices and the latest regulatory requirements, optimized for operational excellence. The Engineering, Procurement, Construction, and Validation(EPCV) Team uses historical data from past projects for feasibility studies, gap analyses, and capital expenditure estimations to deliver customized solutions that meet clients’ needs.
Innovative strategies for enhancing efficiency
Samsung Biologics will adopt the standardized design at Bio Campus II, starting with Plant 5, to enable seamless scaling across multiple projects. This modular manufacturing model will accelerate construction timelines, enhance operational efficiency, and allow for agile adaptation to varying project requirements. By replicating proven designs and processes, Samsung Biologics can scale operations efficiently while maintaining the highest standards of quality and reliability.
To be more flexible and efficient, Samsung Biologics employs an optimized downstream processing strategy that enables the simultaneous processing of multiple batches or different products. This is especially efficient for complex molecules or products that require longer processing times. Minimizing bottlenecks and ensuring continuous operation enables agile responses to changing production demands, maximizing throughput while ensuring quality and speed to market for clients.
“Having manufactured thousands of batches and developed hundreds of products, we have built a robust knowledge base that helps us anticipate challenges and develop cost-effective solutions,” explains Yujin Kim, an engineer in our Engineering Planning Group at Samsung Biologics. “Our focus on continuous improvement and innovation ensures that we are always advancing our capabilities to better support clients.”
Commitment to quality and client success
Samsung Biologics emphasizes quality excellence as well as client satisfaction, and we continue to build on our facility development capabilities to exceed industry standards. The company is committed to providing clients with the utmost flexibility and reliability so they can quickly bring life-saving medicines to patients worldwide.
Samsung Biologics remains steadfast in its commitment to enhance manufacturing capabilities and provide innovative technological solutions. Additionally, the company will closely monitor its clients’ diverse needs and evolving market trends for timely capacity expansion and tailored services.
For more information about Samsung Biologics’ biomanufacturing capabilities, please contact us.
Related contents

Biopharmaceutical manufacturing requires specialized facilities that meet strict regulatory standards to ensure product quality and patient safety. These facilities must be versatile enough to handle unique and complex molecules, including multispecific antibodies, antibody-drug conjugates, and mRNA therapies. Each product type requires highly compatible equipment, environmental controls, and manufacturing processes, adding complexity to facility design and construction.
Samsung Biologics aims to build flexible and efficient facilities to meet each client’s needs. “Our goal is to help our clients achieve faster time to market and reduce costs while maintaining the highest standards of quality,” says Junghyun Bak, Director of Engineering Planning at Samsung Biologics. “By using advanced engineering tools and leveraging our extensive experience, we streamline the entire facility development process.”
Proven track record and expertise in facility design and engineering
From years of experience, Samsung Biologics has refined its facility design methodology to minimize inefficiencies, reduce costs, and shorten project timelines. Our comprehensive strategy encompasses all facility development phases—from design and construction to operational readiness—ensuring each manufacturing site is ready to efficiently produce high-quality biopharmaceutical products.
Samsung Biologics successfully completed four large-scale plants, demonstrating a strong ability to manage complex projects effectively. The company is currently building Plant 5, the first facility of Bio Campus II, whichwill be operational by April 2025. The construction timeline is expected to be approximately 30% shorter than the 35 months taken to complete Plant 3, which has the same capacity. The shortened timeline underscores the company’s expertise and commitment to expanding manufacturing capabilities to meet diverse and immediate client needs.

Advanced engineering and estimation tools are crucial for meticulously planning facility development, including material and utility requirements, project timelines, and cost estimates. Using these tools and a project management information system for real-time management, Samsung Biologics significantly reduced the risk of cost overruns and delays, allowing for efficient resource allocation, benefiting the company and our clients.
Samsung Biologics continuously improves facility design, construction, and validation methodologies. Each new plant implements best practices and the latest regulatory requirements, optimized for operational excellence. The Engineering, Procurement, Construction, and Validation(EPCV) Team uses historical data from past projects for feasibility studies, gap analyses, and capital expenditure estimations to deliver customized solutions that meet clients’ needs.
Innovative strategies for enhancing efficiency
Samsung Biologics will adopt the standardized design at Bio Campus II, starting with Plant 5, to enable seamless scaling across multiple projects. This modular manufacturing model will accelerate construction timelines, enhance operational efficiency, and allow for agile adaptation to varying project requirements. By replicating proven designs and processes, Samsung Biologics can scale operations efficiently while maintaining the highest standards of quality and reliability.

To be more flexible and efficient, Samsung Biologics employs an optimized downstream processing strategy that enables the simultaneous processing of multiple batches or different products. This is especially efficient for complex molecules or products that require longer processing times. Minimizing bottlenecks and ensuring continuous operation enables agile responses to changing production demands, maximizing throughput while ensuring quality and speed to market for clients.
“Having manufactured thousands of batches and developed hundreds of products, we have built a robust knowledge base that helps us anticipate challenges and develop cost-effective solutions,” explains Yujin Kim, an engineer in our Engineering Planning Group at Samsung Biologics. “Our focus on continuous improvement and innovation ensures that we are always advancing our capabilities to better support clients.”
Commitment to quality and client success
Samsung Biologics emphasizes quality excellence as well as client satisfaction, and we continue to build on our facility development capabilities to exceed industry standards. The company is committed to providing clients with the utmost flexibility and reliability so they can quickly bring life-saving medicines to patients worldwide.
Samsung Biologics remains steadfast in its commitment to enhance manufacturing capabilities and provide innovative technological solutions. Additionally, the company will closely monitor its clients’ diverse needs and evolving market trends for timely capacity expansion and tailored services.
For more information about Samsung Biologics’ biomanufacturing capabilities, please contact us.
Related contents
Share article
Related Content