Samsung Biologics’ Super Plant 4, How the world’s largest biomanufacturing plant will drive clients’ success

This month, Samsung Biologics commenced GMP operations at its newest Plant 4. With the world’s largest contract manufacturing capacity, the company plans to maximize operational efficiency for its clients and further strengthen its foothold in the industry as an end-to-end service partner.
Breaking world records
In just 23 months since its groundbreaking in November 2020, Samsung Biologics delivered on its commitment to begin partial operations at Plant 4.
As the world’s largest single biomanufacturing plant, Plant 4 is expected to have a production capacity of 240,000 liters upon full completion next year, giving Samsung Biologics a total of 604,000 liters’ capacity, amounting to over a quarter of the total global CDMO production, at Bio Campus I.

Plant 4 groundbreaking ceremony (Nov. 2020)
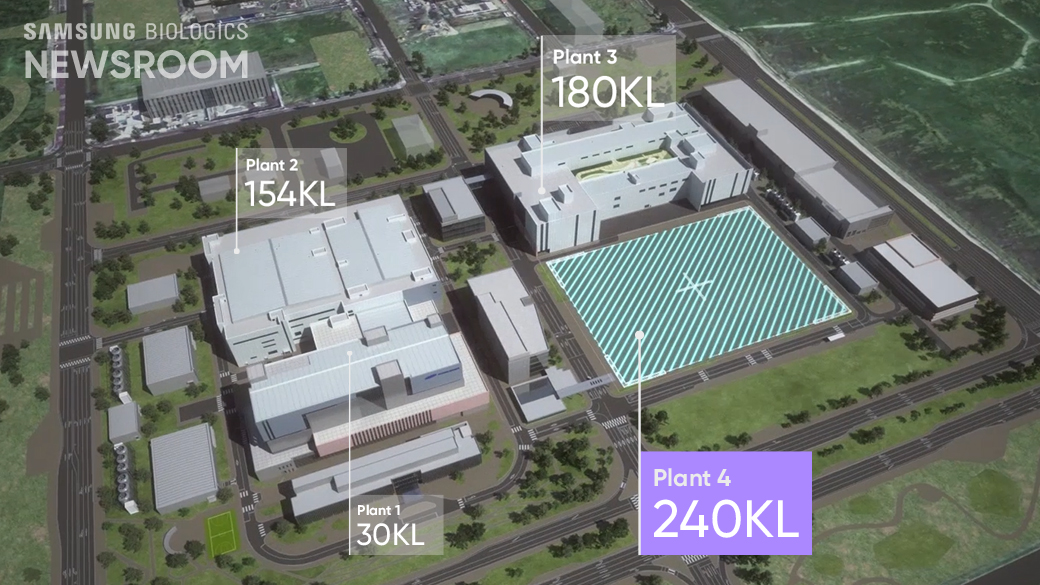
Samsung Biologics’ total manufacturing capacity
Samsung Biologics offers end-to-end services, from early stage development to commercial manufacturing, including quality management. The company also has a diverse lineup of bioreactors, from 1,000 liters to 15,000 liters, enabling flexibility for small batch clinical trial materials production or large-scale commercial manufacturing.
Samsung Biologics can swiftly and efficiently manufacture high-quality biopharmaceuticals based on N-1 perfusion technology, data digitalization and cyber security integrity. Plant 4 incorporates the know-how, technologies and best practices learned at Plants 1, 2 and 3.
Investing in a sustainable tomorrow
As a sustainable CDMO, Samsung Biologics invested in eco-friendly technology throughout Plant 4 to mitigate its emissions. A 1,300 kW solar power generator was installed on the plant’s roof over an area of 4,000㎡ to partially generate energy for the plant.
In order to reduce direct GHG emissions, the company adopted the ‘Factory Energy Management System,’ to monitor and analyze energy consumption to help reduce emissions and waste. Through its environmental management strategy, the company plans to take additional measures going forward to enhance its recycling process when it comes to disposables and water use, reduce energy consumption by switching to high-efficiency boilers, and prevent air pollution to help tackle climate change.

Solar panels installed on Plant 4
Accelerating excellence
Plant 4 can handle a product’s entire production life cycle within a single site. It will also be equipped with multiple digital solutions to achieve data integrity and transparency, while eliminating the potential of human error.
Samsung Biologics plans to further boost client satisfaction through continuous innovation and investments, as well as the construction of Bio Campus II, which will host Plants 5 through 8. Samsung Biologics will continue to accelerate excellence for a healthier future and bolster its standing as a leading CDMO by expanding its capacity, portfolio and global presence.



Opening ceremony of Plant 4 (Oct. 2022)
Related Content
YouTube The Future of Biopharma: Plant 4
YouTube Samsung Biologics Plant 4: Specs & Features

This month, Samsung Biologics commenced GMP operations at its newest Plant 4. With the world’s largest contract manufacturing capacity, the company plans to maximize operational efficiency for its clients and further strengthen its foothold in the industry as an end-to-end service partner.
Breaking world records
In just 23 months since its groundbreaking in November 2020, Samsung Biologics delivered on its commitment to begin partial operations at Plant 4.
As the world’s largest single biomanufacturing plant, Plant 4 is expected to have a production capacity of 240,000 liters upon full completion next year, giving Samsung Biologics a total of 604,000 liters’ capacity, amounting to over a quarter of the total global CDMO production, at Bio Campus I.

Plant 4 groundbreaking ceremony (Nov. 2020)

Samsung Biologics’ total manufacturing capacity
Samsung Biologics offers end-to-end services, from early stage development to commercial manufacturing, including quality management. The company also has a diverse lineup of bioreactors, from 1,000 liters to 15,000 liters, enabling flexibility for small batch clinical trial materials production or large-scale commercial manufacturing.
Samsung Biologics can swiftly and efficiently manufacture high-quality biopharmaceuticals based on N-1 perfusion technology, data digitalization and cyber security integrity. Plant 4 incorporates the know-how, technologies and best practices learned at Plants 1, 2 and 3.
Investing in a sustainable tomorrow
As a sustainable CDMO, Samsung Biologics invested in eco-friendly technology throughout Plant 4 to mitigate its emissions. A 1,300 kW solar power generator was installed on the plant’s roof over an area of 4,000㎡ to partially generate energy for the plant.
In order to reduce direct GHG emissions, the company adopted the ‘Factory Energy Management System,’ to monitor and analyze energy consumption to help reduce emissions and waste. Through its environmental management strategy, the company plans to take additional measures going forward to enhance its recycling process when it comes to disposables and water use, reduce energy consumption by switching to high-efficiency boilers, and prevent air pollution to help tackle climate change.

Solar panels installed on Plant 4
Accelerating excellence
Plant 4 can handle a product’s entire production life cycle within a single site. It will also be equipped with multiple digital solutions to achieve data integrity and transparency, while eliminating the potential of human error.
Samsung Biologics plans to further boost client satisfaction through continuous innovation and investments, as well as the construction of Bio Campus II, which will host Plants 5 through 8. Samsung Biologics will continue to accelerate excellence for a healthier future and bolster its standing as a leading CDMO by expanding its capacity, portfolio and global presence.



Opening ceremony of Plant 4 (Oct. 2022)
Related Content
YouTube The Future of Biopharma: Plant 4
YouTube Samsung Biologics Plant 4: Specs & Features
- CDO
- CGMP
- ADC
- Bio Campus
- IR
- CMO
Share article