The Journey to Your Success | Explore Samsung Biologics' CDO Strengths

Biologics are complex, large-molecule substances* that require controlled processes and up-to-date technology to develop. An excellent understanding of the scientific, logistical, and regulatory challenges is also essential for the successful development of biologics to reach product approvals before going into large-scale manufacturing.
With considerable challenges to safely and effectively develop biologics, many biopharmaceutical companies are choosing to outsource their development processes to CDO partners. In response to the increasing demand of biologics, Samsung Biologics now not only holds the world’s largest biomanufacturing capacity, but also offers contract development services, establishing the company as a one-stop CDMO service provider. In October 2020, Samsung Biologics opened its first R&D Center in San Francisco, at the heart of the leading U.S. bio-cluster, to be in close proximity to clients as the next door CDO partner.
In this article, we will explore the services a CDO partner provides and the unique capabilities Samsung Biologics can bring for a molecule’s success.
*Refer to “A New Era in Biotechnology: What are Biomedicines?”
Contract Development Services to Expedite Success for Your Molecule
The development of new drugs is divided into two different stages: the ‘discovery’ stage where a candidate molecule for a specific disease is discovered and the ‘development’ stage which includes all the processes that are required to reach clinical trials and gain product approvals. Contract Development Organizations (CDO) provide the ‘development’ part of the service required for the overall development of new drugs.
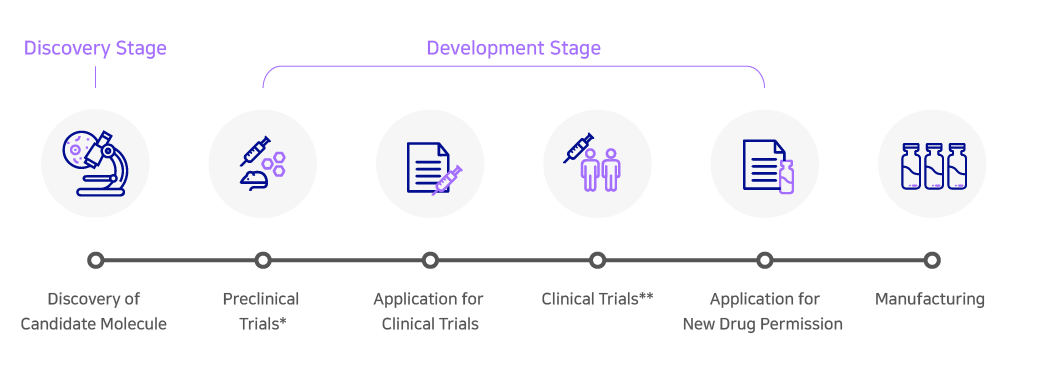
*Preclinical Trials: Research using animals to find out if a drug, procedure, or treatment is likely to be useful
**Clinical Trials: Research studies performed in people to test the stability and safety of a new candidate molecule
Drug development is a costly process that takes on average more than a decade from discovery to approval. Both the costs and timelines still continue to rise with the increased identification of novel targets and their associated modalities.
Due to the huge investment that goes into the development of a single drug, biopharmaceutical companies have the potential to become market dominant upon their application for patents of the product. Hence the order of market entry is a critical factor to market share with the first and second products commercialized accounting for over 70%. As such, a faster timeline to develop a new drug is important now more than ever.
Major biopharmaceutical companies are opting to outsource the development processes to CDO partners to expedite the timeline to market and enhance efficiency. By outsourcing to a CDO partner like Samsung Biologics, biopharmaceutical companies, no matter the size, are able to gain access to top-notch R&D capabilities, up-to-date technology, and teams of experts to ensure both the quality and speed of their molecules.
The Journey to a Successful Development of Biologics
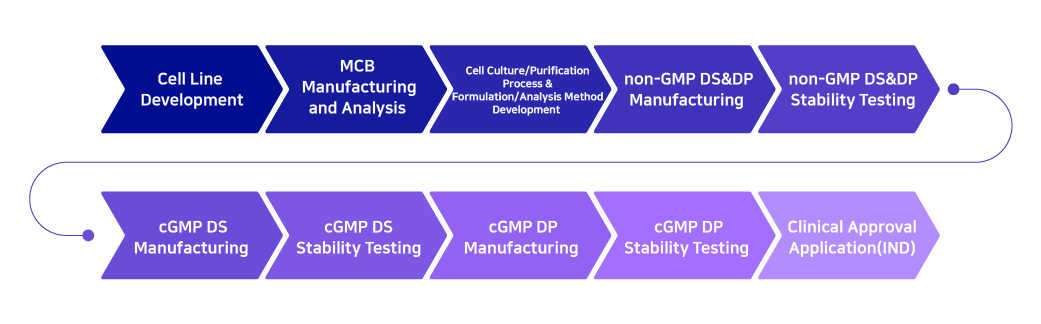
Once a client discovers an initial candidate molecule and a contract development agreement is made, cell line development begins. A cell line is a permanently established cell culture that will proliferate indefinitely given the appropriate fresh medium and space to ultimately produce antibodies for biomedicines.
Upon completion of development for a high-quality cell line, a CDO partner begins the development of ‘process, formulation, and analysis’ which is followed up by ‘non-GMP Drug substance and Drug Product’ manufacturing that are needed for preclinical trials. Once the manufacturing and stability testing are completed, submission for IND approval is done.
Faster and Better CDO Services
‘Technology’ and ‘speed’ are key competitive advantages that Samsung Biologics offers. In August 2020, Samsung Biologics introduced its own proprietary cell line, S-CHOice, offering a wide array of choices for its clients. S-CHOice builds on glutamine synthase (GS) knock-out Chinese hamster ovary (CHO) cell line technology and shows improved titers up to two-fold from industry average, reaching above 7 g/L for standard monoclonal antibodies. The cell line also shows enhanced cell viability with over 90% at day-21 in a fed-batch study, demonstrating effectiveness in producing high quality cell lines. The longer the cell viability, the better chances for selecting high-quality cell line for large-scale manufacturing, which improves the overall productivity.
Samsung Biologics has the capability to offer contract manufacturing services at a single site at its Songdo headquarters. Upon successful clinical trial, clients are able to gain access to its manufacturing expertise and world-class facilities with the largest biomanufacturing capacity to successfully bring the final products to patients. The company offers a differentiated process of contract manufacturing through its unique capabilities as a fully-integrated CDMO partner while ensuring both quality, stability, and speed of products to save lives of patients.
Samsung Biologics has also implemented its latest CDO service platform at the San Francisco R&D Center to be in close proximity to global clients and provide real-time interactive services. The company also plans to expand geographically in Boston and Europe to offer enhanced convenience to the clients for their drug development processes.
From Gene to Commercialization, Your Partner for Success
Samsung Biologics, which started its business as a contract manufacturing organization (CMO), has expanded its business portfolio in response to the rapidly increasing demand globally for contract development services and is now recognized as a global CDMO company with the world’s largest biomanufacturing capacity.
We will continue to offer customized solutions for our clients with flexibility and enhance our capabilities to successfully respond to the global trends.
Samsung Biologics will support the development of new drugs faster through its one-stop service, strive to build a virtuous cycle of bio-ecosystems, and save lives of patients who are in urgent need.
Related Contents
Samsung Bio Insight The Benefit of Speed, Scale & Quality | Explore Samsung Biologics' CMO Strengths
Webinars What to consider in selecting the best cell line to accelerate the timeline to IND

Biologics are complex, large-molecule substances* that require controlled processes and up-to-date technology to develop. An excellent understanding of the scientific, logistical, and regulatory challenges is also essential for the successful development of biologics to reach product approvals before going into large-scale manufacturing.
With considerable challenges to safely and effectively develop biologics, many biopharmaceutical companies are choosing to outsource their development processes to CDO partners. In response to the increasing demand of biologics, Samsung Biologics now not only holds the world’s largest biomanufacturing capacity, but also offers contract development services, establishing the company as a one-stop CDMO service provider. In October 2020, Samsung Biologics opened its first R&D Center in San Francisco, at the heart of the leading U.S. bio-cluster, to be in close proximity to clients as the next door CDO partner.
In this article, we will explore the services a CDO partner provides and the unique capabilities Samsung Biologics can bring for a molecule’s success.
*Refer to “A New Era in Biotechnology: What are Biomedicines?”
Contract Development Services to Expedite Success for Your Molecule
The development of new drugs is divided into two different stages: the ‘discovery’ stage where a candidate molecule for a specific disease is discovered and the ‘development’ stage which includes all the processes that are required to reach clinical trials and gain product approvals. Contract Development Organizations (CDO) provide the ‘development’ part of the service required for the overall development of new drugs.

*Preclinical Trials: Research using animals to find out if a drug, procedure, or treatment is likely to be useful
**Clinical Trials: Research studies performed in people to test the stability and safety of a new candidate molecule
Drug development is a costly process that takes on average more than a decade from discovery to approval. Both the costs and timelines still continue to rise with the increased identification of novel targets and their associated modalities.
Due to the huge investment that goes into the development of a single drug, biopharmaceutical companies have the potential to become market dominant upon their application for patents of the product. Hence the order of market entry is a critical factor to market share with the first and second products commercialized accounting for over 70%. As such, a faster timeline to develop a new drug is important now more than ever.
Major biopharmaceutical companies are opting to outsource the development processes to CDO partners to expedite the timeline to market and enhance efficiency. By outsourcing to a CDO partner like Samsung Biologics, biopharmaceutical companies, no matter the size, are able to gain access to top-notch R&D capabilities, up-to-date technology, and teams of experts to ensure both the quality and speed of their molecules.
The Journey to a Successful Development of Biologics

Once a client discovers an initial candidate molecule and a contract development agreement is made, cell line development begins. A cell line is a permanently established cell culture that will proliferate indefinitely given the appropriate fresh medium and space to ultimately produce antibodies for biomedicines.
Upon completion of development for a high-quality cell line, a CDO partner begins the development of ‘process, formulation, and analysis’ which is followed up by ‘non-GMP Drug substance and Drug Product’ manufacturing that are needed for preclinical trials. Once the manufacturing and stability testing are completed, submission for IND approval is done.
Faster and Better CDO Services
‘Technology’ and ‘speed’ are key competitive advantages that Samsung Biologics offers. In August 2020, Samsung Biologics introduced its own proprietary cell line, S-CHOice, offering a wide array of choices for its clients. S-CHOice builds on glutamine synthase (GS) knock-out Chinese hamster ovary (CHO) cell line technology and shows improved titers up to two-fold from industry average, reaching above 7 g/L for standard monoclonal antibodies. The cell line also shows enhanced cell viability with over 90% at day-21 in a fed-batch study, demonstrating effectiveness in producing high quality cell lines. The longer the cell viability, the better chances for selecting high-quality cell line for large-scale manufacturing, which improves the overall productivity.
Samsung Biologics has the capability to offer contract manufacturing services at a single site at its Songdo headquarters. Upon successful clinical trial, clients are able to gain access to its manufacturing expertise and world-class facilities with the largest biomanufacturing capacity to successfully bring the final products to patients. The company offers a differentiated process of contract manufacturing through its unique capabilities as a fully-integrated CDMO partner while ensuring both quality, stability, and speed of products to save lives of patients.
Samsung Biologics has also implemented its latest CDO service platform at the San Francisco R&D Center to be in close proximity to global clients and provide real-time interactive services. The company also plans to expand geographically in Boston and Europe to offer enhanced convenience to the clients for their drug development processes.

From Gene to Commercialization, Your Partner for Success
Samsung Biologics, which started its business as a contract manufacturing organization (CMO), has expanded its business portfolio in response to the rapidly increasing demand globally for contract development services and is now recognized as a global CDMO company with the world’s largest biomanufacturing capacity.
We will continue to offer customized solutions for our clients with flexibility and enhance our capabilities to successfully respond to the global trends.
Samsung Biologics will support the development of new drugs faster through its one-stop service, strive to build a virtuous cycle of bio-ecosystems, and save lives of patients who are in urgent need.
Related Contents
Samsung Bio Insight The Benefit of Speed, Scale & Quality | Explore Samsung Biologics' CMO Strengths
Webinars What to consider in selecting the best cell line to accelerate the timeline to IND
- CDO
- CGMP
- ADC
- Bio Campus
- IR
- CMO
Share article