Samsung Biologics expands global footprint with opening of new San Francisco CDO R&D Center
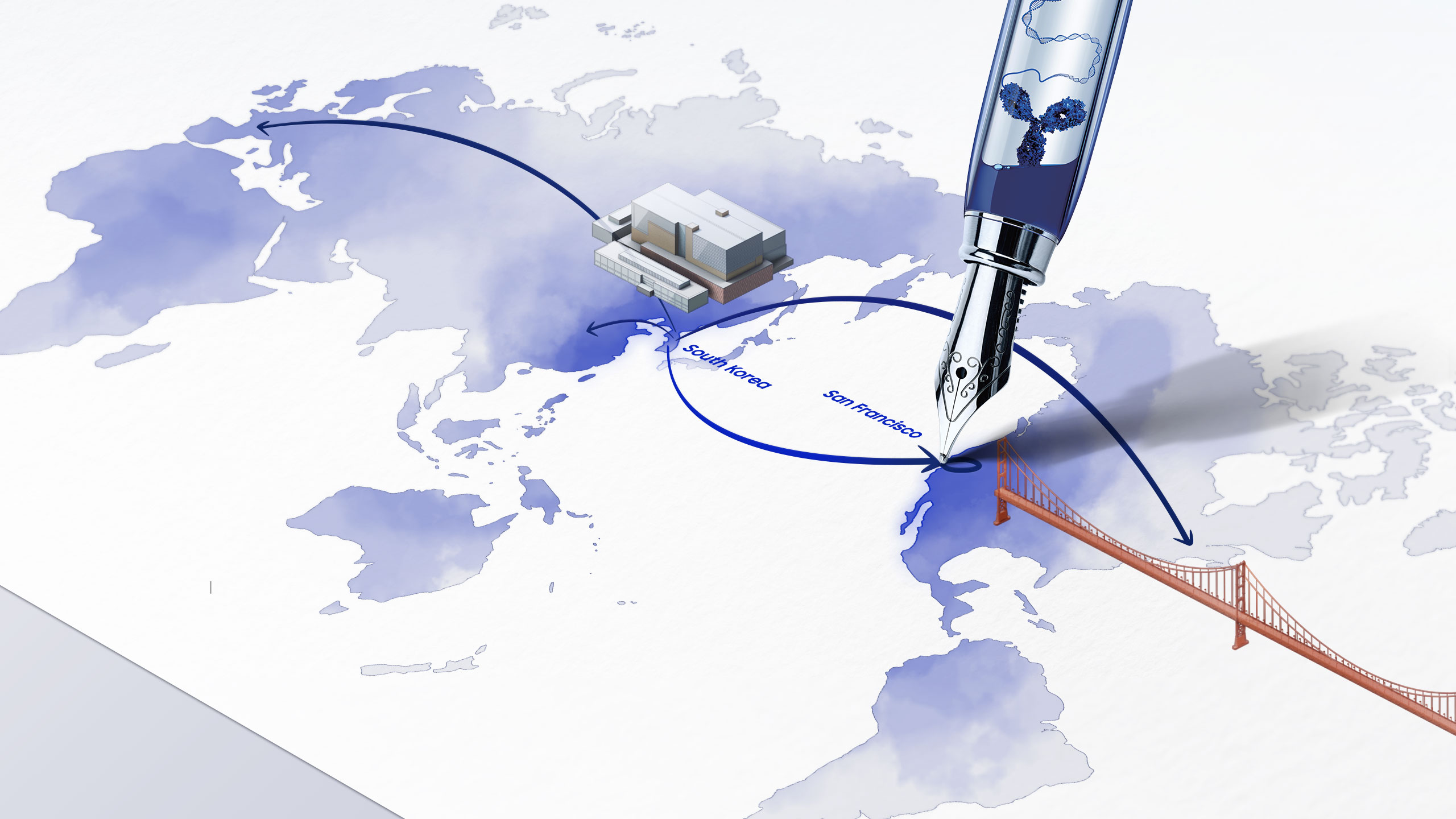
INCHEON, S. KOREA and SAN FRANCISCO, US, 28 October 2020 – At a virtual ceremony today, Samsung Biologics (KRX: 207940.KS) announced the grand opening of its first US CDO R&D Center in San Francisco, bringing its contract development services closer to global clients. As the “Next Door CDO Partner,” the Company aims to maximize client satisfaction and provide immediate convenience to biotech companies in the Bay area.
Tapping into San Francisco, the birthplace of biotech and one of the fastest developing bioclusters, Samsung Biologics will provide real-time interaction and be in closer proximity to potential and existing clients in the region. Aligned with the Company’s mission to offer a seamless one-stop service, the new facility will utilize the same CDO research and development capabilities present at its headquarters in Incheon, South Korea. Samsung Biologics’ CDO business brings quality-driven development services at a greater speed, delivering cell line development to DS manufacturing in six months, and to DP manufacturing in seven months at the fastest pace in the industry.
“We’re starting the CDO R&D Center today in San Francisco, and we plan to continue our expansion into Boston, Europe, and China moving forward. In 2020 and beyond, our next decade will be marked by global geographical expansion,” said Tae Han Kim, CEO of Samsung Biologics. “Samsung Biologics will continue to provide biopharmaceutical development and manufacturing services with top-notch capabilities and capacity, so that biotech and pharmaceutical companies can focus on their expertise: the discovery, development, or marketing and sales of novel biologics for patients.”
Recognized as a CMO Champion for its excellence in capabilities and reliability, its goal is to become CDO champion by 2025. Since its launch, the Samsung Biologics’ CDO business has been growing at a fast rate, contracting nearly 60 projects within just two years and establishing its proprietary cell-line, S-CHOice, showing improved titers up to two-fold from the industry average and maintaining over 90% cell viability.
Leveraging the Company’s operational excellence, Samsung Biologics plans to go above and beyond by offering end-to-end service from the early stages of discovery to the final manufacturing of drug products in a shorter development time.
With its experts working “next-door,” the newly opened San Francisco CDO R&D Center will bridge Samsung Biologics with its biotech clients in its first step towards global expansion in providing optimized satisfaction and even greater convenience with faster and better service offerings.
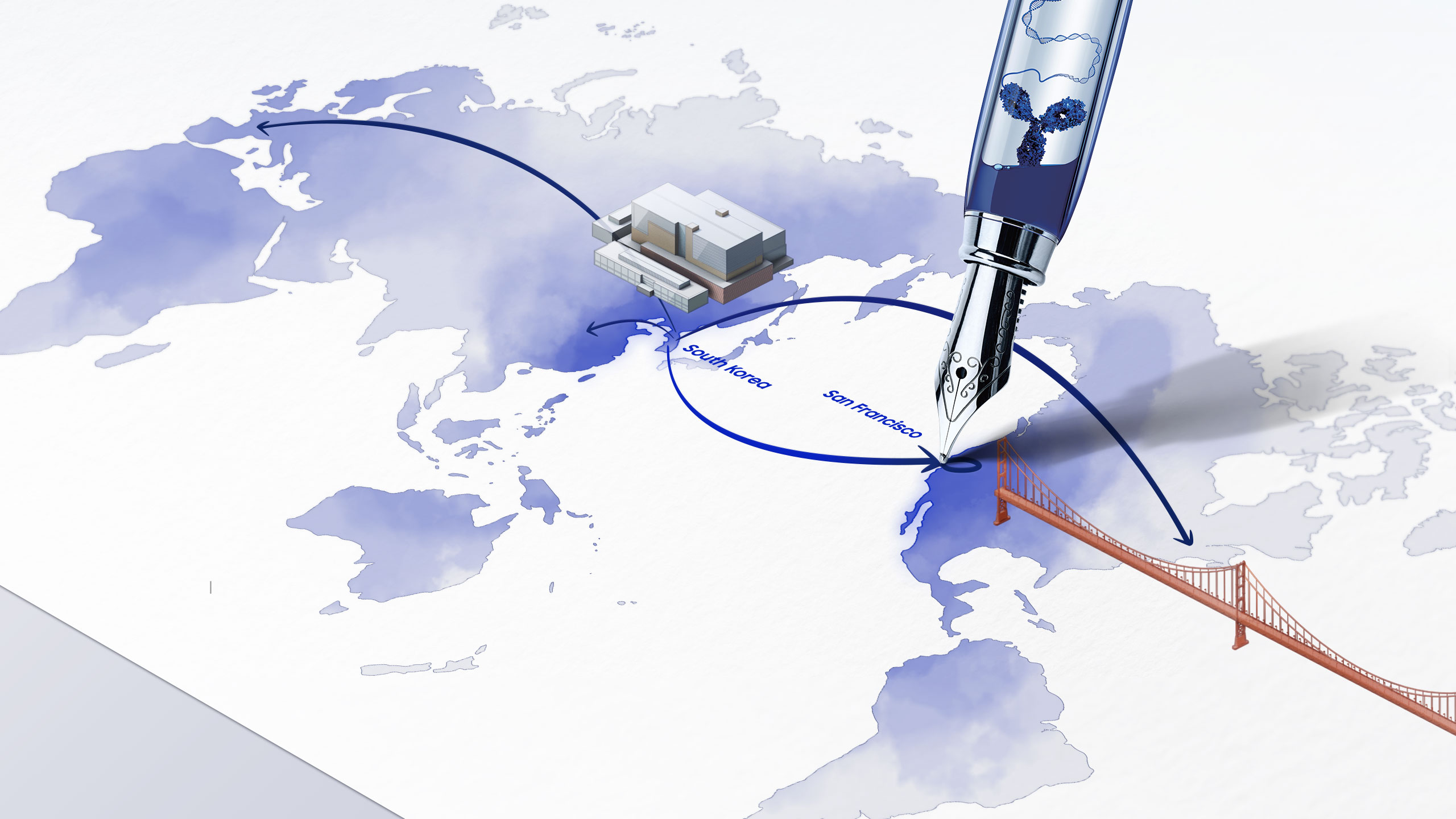
INCHEON, S. KOREA and SAN FRANCISCO, US, 28 October 2020 – At a virtual ceremony today, Samsung Biologics (KRX: 207940.KS) announced the grand opening of its first US CDO R&D Center in San Francisco, bringing its contract development services closer to global clients. As the “Next Door CDO Partner,” the Company aims to maximize client satisfaction and provide immediate convenience to biotech companies in the Bay area.
Tapping into San Francisco, the birthplace of biotech and one of the fastest developing bioclusters, Samsung Biologics will provide real-time interaction and be in closer proximity to potential and existing clients in the region. Aligned with the Company’s mission to offer a seamless one-stop service, the new facility will utilize the same CDO research and development capabilities present at its headquarters in Incheon, South Korea. Samsung Biologics’ CDO business brings quality-driven development services at a greater speed, delivering cell line development to DS manufacturing in six months, and to DP manufacturing in seven months at the fastest pace in the industry.
“We’re starting the CDO R&D Center today in San Francisco, and we plan to continue our expansion into Boston, Europe, and China moving forward. In 2020 and beyond, our next decade will be marked by global geographical expansion,” said Tae Han Kim, CEO of Samsung Biologics. “Samsung Biologics will continue to provide biopharmaceutical development and manufacturing services with top-notch capabilities and capacity, so that biotech and pharmaceutical companies can focus on their expertise: the discovery, development, or marketing and sales of novel biologics for patients.”
Recognized as a CMO Champion for its excellence in capabilities and reliability, its goal is to become CDO champion by 2025. Since its launch, the Samsung Biologics’ CDO business has been growing at a fast rate, contracting nearly 60 projects within just two years and establishing its proprietary cell-line, S-CHOice, showing improved titers up to two-fold from the industry average and maintaining over 90% cell viability.
Leveraging the Company’s operational excellence, Samsung Biologics plans to go above and beyond by offering end-to-end service from the early stages of discovery to the final manufacturing of drug products in a shorter development time.
With its experts working “next-door,” the newly opened San Francisco CDO R&D Center will bridge Samsung Biologics with its biotech clients in its first step towards global expansion in providing optimized satisfaction and even greater convenience with faster and better service offerings.
- CDO
- CGMP
- ADC
- Bio Campus
- IR
- CMO
Share article